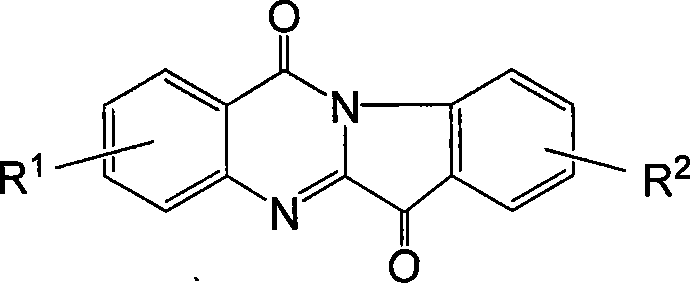
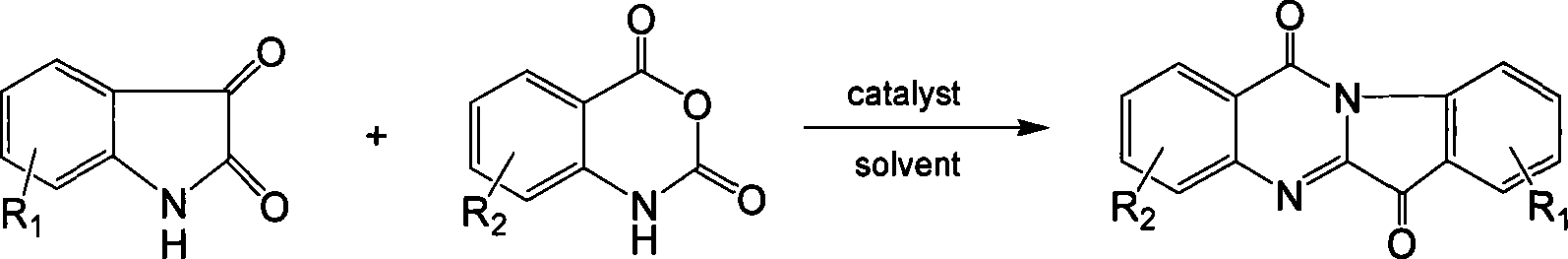
One-step method for synthesizing tryptamine ketone and derivative
A synthesis method and a derivative technology are applied in the synthesis field of tryptamine and its derivatives, which can solve the problems of difficulty in large-scale production, unpleasant smell and the like, and achieve the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of tryptanthrin
[0029] Dissolve equimolar indoloquinone and isatoic anhydride in 1,2-dichloroethane, add tributylamine, heat to reflux for 2 hours, evaporate the solvent and catalyst after cooling. The target compound tryptanthrin was obtained after recrystallization from ethanol with a yield of 92%.
Embodiment 2
[0030] Embodiment 2: the synthesis of 8-chlorotryptanthrin
[0031] Dissolve equimolar 5-chloroindoloquinone and isatoic anhydride in carbon tetrachloride, add N,N-dipropylbutylamine, heat and reflux for 3 hours, and evaporate the solvent and catalyst after cooling. The target compound 8-chlorotryptanthin was obtained after recrystallization from ethanol with a yield of 90%.
Embodiment 3
[0032] Example 3: Synthesis of 2-ethoxyl-8-methoxytryptanthrin
[0033] Dissolve equimolar 5-methoxyindolequinone and 6-ethoxyisatoic anhydride in n-propanol, add sodium n-propoxide, heat and reflux for 2.5 hours, add dilute hydrochloric acid to neutralize after cooling, evaporate solvent. The target compound 2-ethoxy-8-methoxytryptanthrin was obtained after recrystallization from ethanol with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com