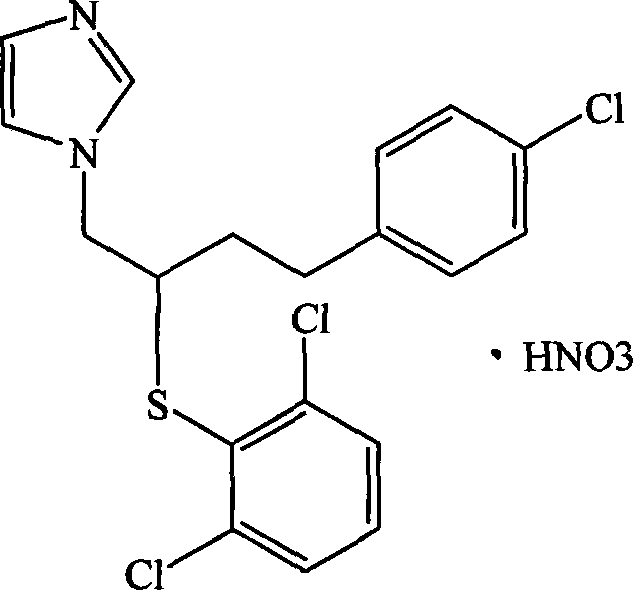
Nitric acid butoconazole cyclodextrin or cyclodextrin derivant clathrate and preparation method as well as application thereof
A technology of butoconazole nitrate cyclodextrin and butoconazole nitrate, which is applied in the field of medicine, can solve the problems such as the lack of research on the water solubility of butoconazole nitrate, and achieve the effect of safe and effective clinical medication, clear and transparent appearance, and easy to accept
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]Example 1: Preparation of liquid butoconazole nitrate hydroxypropyl-β-cyclodextrin inclusion compound
[0039] (1) Weigh 200g of hydroxypropyl-β-cyclodextrin, pour it into 200ml of water, and stir to dissolve;
[0040] (2) Take another 10g of butoconazole nitrate, pour it into the above-mentioned hydroxypropyl-β-cyclodextrin solution, and stir the mixture for 20 minutes with the method of Zili stirrer, and the stirring speed should be such that the liquid does not splash. Until it becomes clear and transparent, 210 g of butoconazole nitrate hydroxypropyl-β-cyclodextrin inclusion compound is obtained as a light yellow transparent liquid. It has been determined that the clathrate is easily soluble in water.
Embodiment 2
[0041] Example 2: Preparation of solid butoconazole nitrate hydroxypropyl-β-cyclodextrin inclusion compound
[0042] (1) Weigh 200g of hydroxypropyl-β-cyclodextrin, pour it into a mixture of 200ml of water and 40ml of absolute ethanol, and stir to dissolve;
[0043] (2) Take another 10g of butoconazole nitrate, pour it into the above mixed solution, and stir the mixed solution for 20 minutes with a stirrer method, the stirring speed should be such that the liquid does not splash outside, and observe the solution until it is clear and transparent, and liquid nitric acid is obtained. Hydroxypropyl-β-cyclodextrin inclusion complex of butoconazole.
[0044] (3) Concentrate the liquid butoconazole nitrate hydroxypropyl-β-cyclodextrin inclusion compound, and freeze-dry to obtain 210 g of off-white solid butoconazole nitrate hydroxypropyl-β-cyclodextrin inclusion compound. It has been determined that the clathrate is easily soluble in water.
Embodiment 3
[0045] Example 3: Preparation of gel with butoconazole nitrate hydroxypropyl-β-cyclodextrin inclusion compound
[0046] (1) Take butoconazole nitrate 4g;
[0047] (2) Dissolve 80g of hydroxypropyl-β-cyclodextrin in 150ml of water for injection;
[0048] (3) Butoconazole nitrate is added to the hydroxypropyl-β-cyclodextrin solution and stirred for clathrate;
[0049] (4) Add 6.0g of glycerin, 4.0g of ethanol, 6.0g of hypromellose, add water for injection to 200ml, disperse for 5 hours, fill, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com