Pirarubicin freeze-dry preparations and preparation method thereof
A technology for pirarubicin and freeze-dried preparations, which is applied in the field of pirarubicin liposome freeze-dried preparations and their preparation, can solve the problems of poor preparation stability, poor anti-tumor activity, large toxic and side effects of the reticuloendothelial system, etc. problems, to achieve the effect of improving stability, improving anti-tumor activity, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
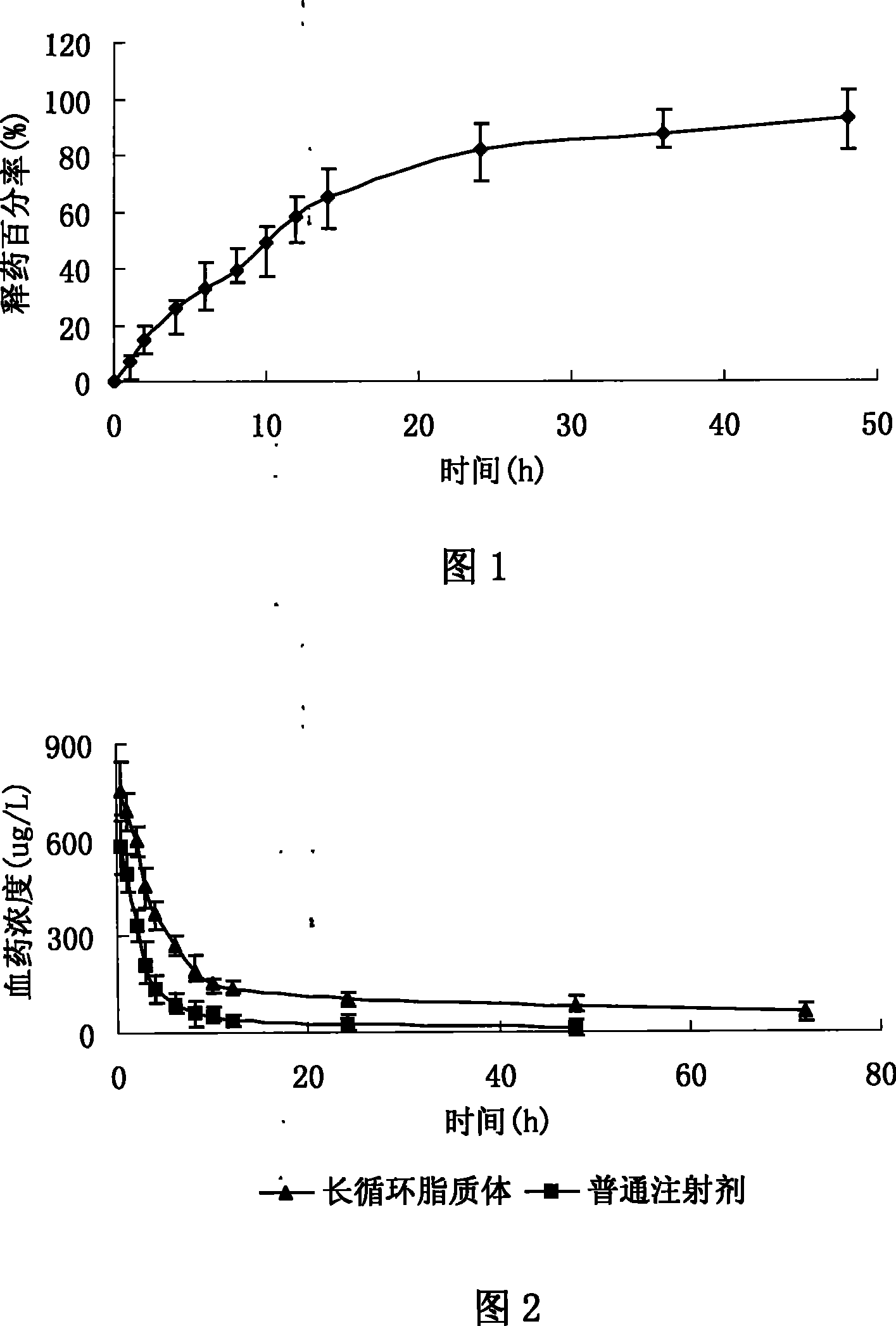
Image
Examples
Embodiment 1
[0030] Pirarubicin 10g
[0031] Hydrogenated Soy Lecithin 63g
[0032] Cholesterol 35g
[0033] PEG2000-Dipalmitoylphosphatidylethanolamine 4g
[0034] Poloxamer 188 1.5g
[0035] a-Tocopherol 40mg
[0036] Sucrose 625g
[0037] Preparation method: Dissolve the prescribed amount of pirarubicin, hydrogenated soybean lecithin, cholesterol, PEG2000-dipalmitoylphosphatidylethanolamine and a-tocopherol in absolute ethanol, remove the absolute ethanol by rotary evaporation to make a uniform lipid film. The lipid film was hydrated with PH7.4 phosphate buffer containing the prescribed amount of poloxamer 188 and sucrose in a constant temperature water bath at 60°C with nitrogen gas for 30 minutes, then transferred to an ice bath to cool, and then was homogenized with a high-pressure milk homogenizer (high-pressure homogenizer). 408kg / cm 2 2 times, low pressure 102kg / cm 2 1 time) Dispersion treatment, filtration with 200nm microporous membrane, and lyophilization in a lyophiliz...
Embodiment 2
[0066] Pirarubicin Hydrochloride 12g
[0067] Hydrogenated Soy Lecithin 75g
[0068] Cholesterol 42g
[0069] PEG2000-Dipalmitoylphosphatidylethanolamine 5g
[0070] Poloxamer 188 2g
[0071] a-Tocopherol 50mg
[0072] Sucrose 900g
[0073] Preparation method: Dissolve the prescribed amount of pirarubicin hydrochloride, hydrogenated soybean lecithin, cholesterol, PEG2000-dipalmitoylphosphatidylethanolamine and a-tocopherol in absolute ethanol, and remove the absolute ethanol by rotary evaporation to make a uniform lipid The lipid film was hydrated with PH6.5 phosphate buffer solution containing the prescribed amount of poloxamer 188 and sucrose, filled with nitrogen in a constant temperature water bath at 50°C for 60 minutes, then transferred to an ice bath to cool, and then homogenized with a high-pressure milk homogenizer. (high pressure 510kg / cm 2 2 times, low pressure 102kg / cm 2 1 time) Dispersion treatment, filtration with 100nm microporous membrane, and lyophiliza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com