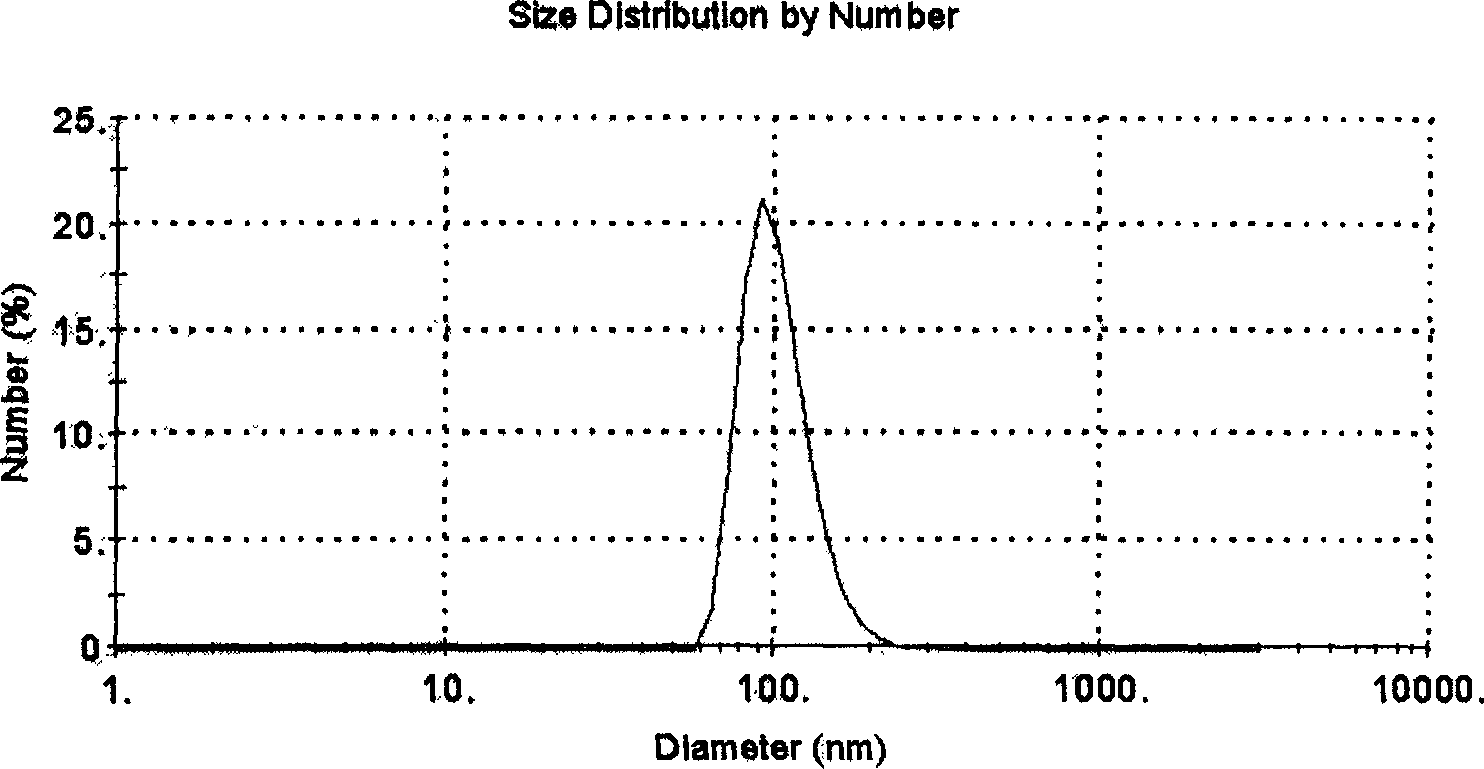
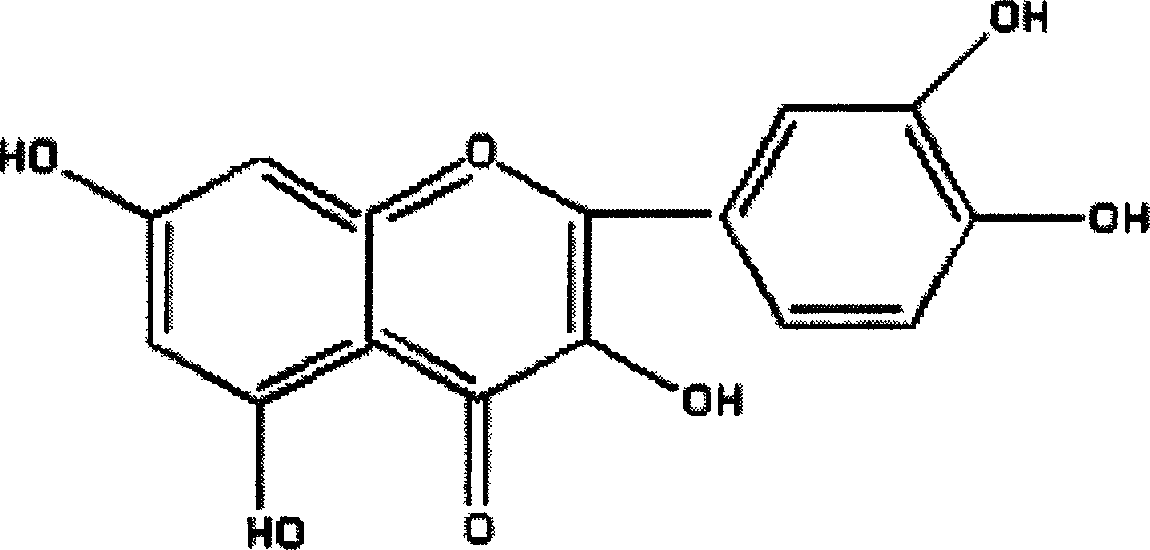
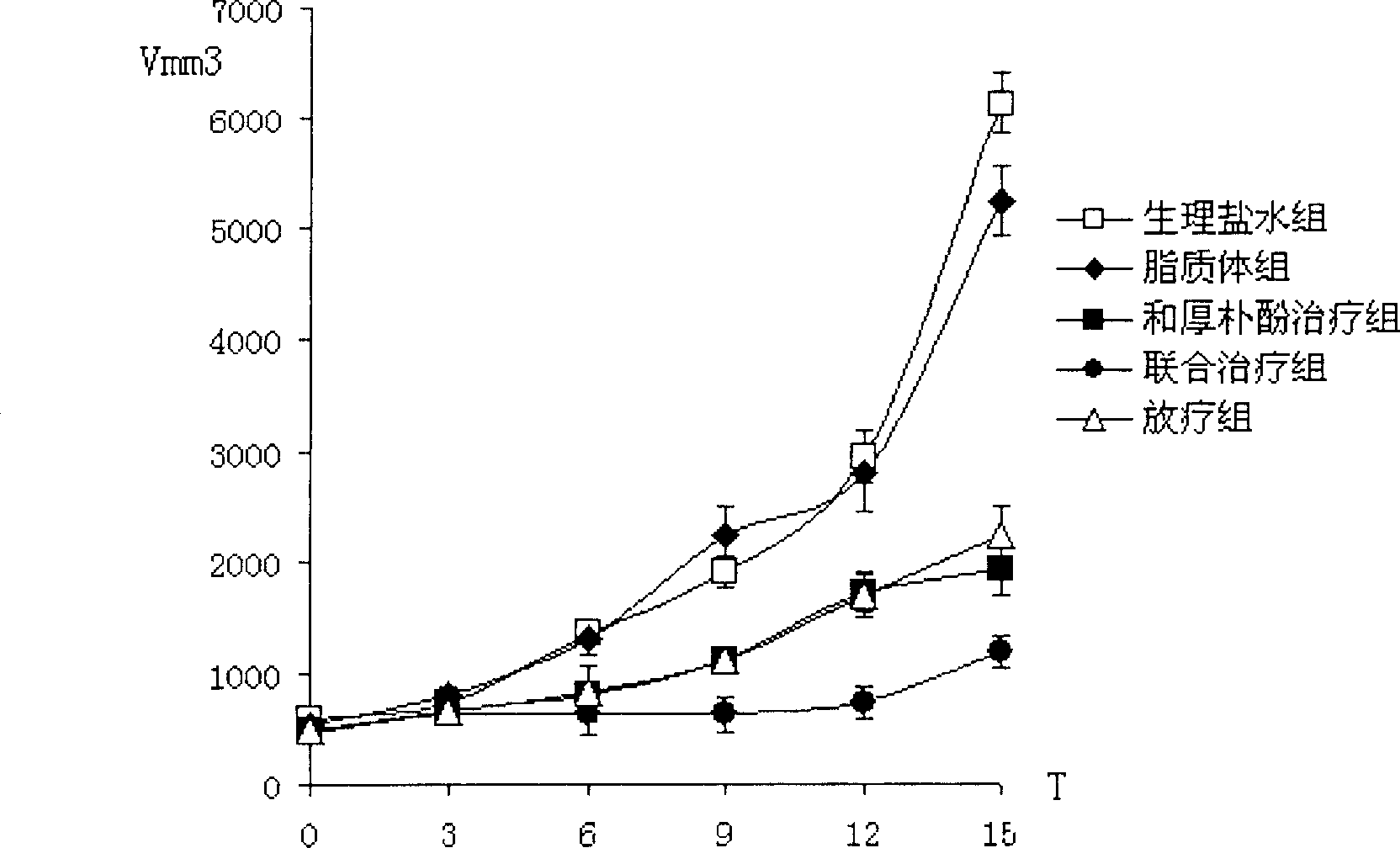
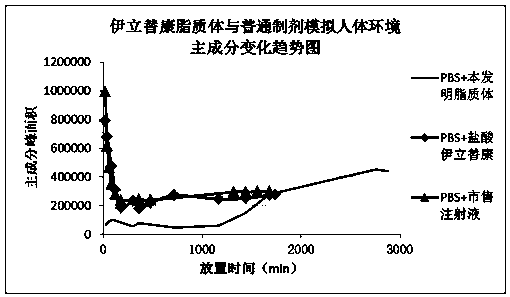
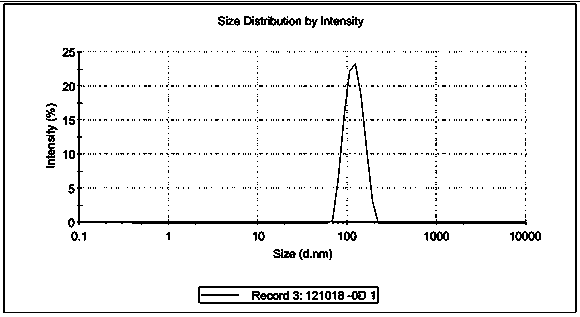
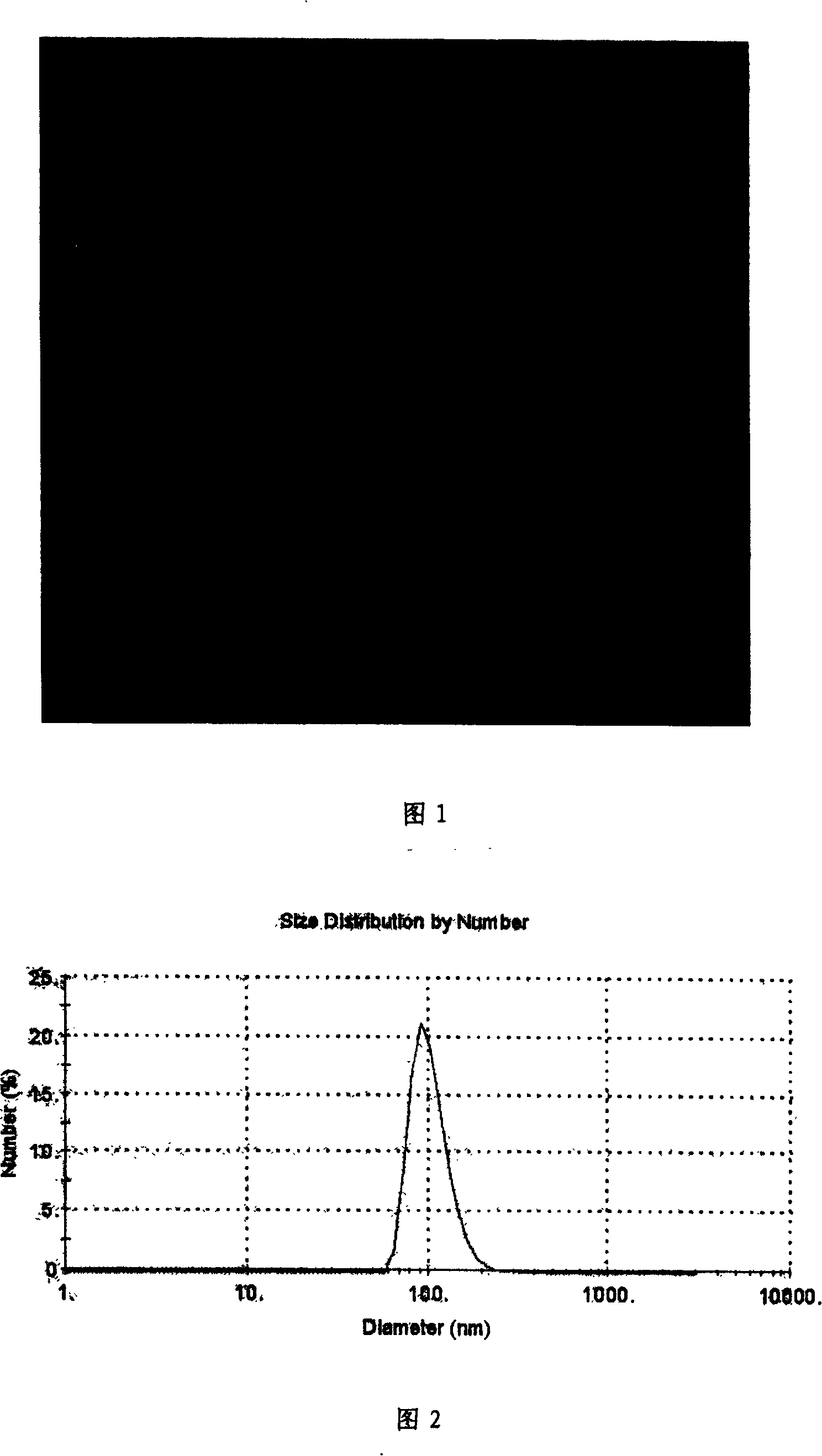
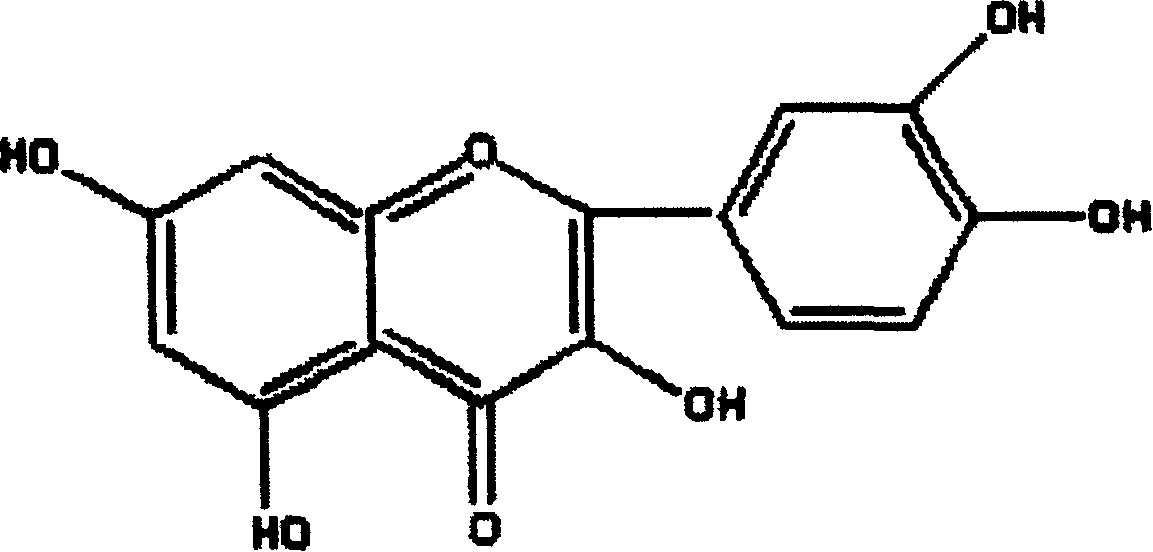
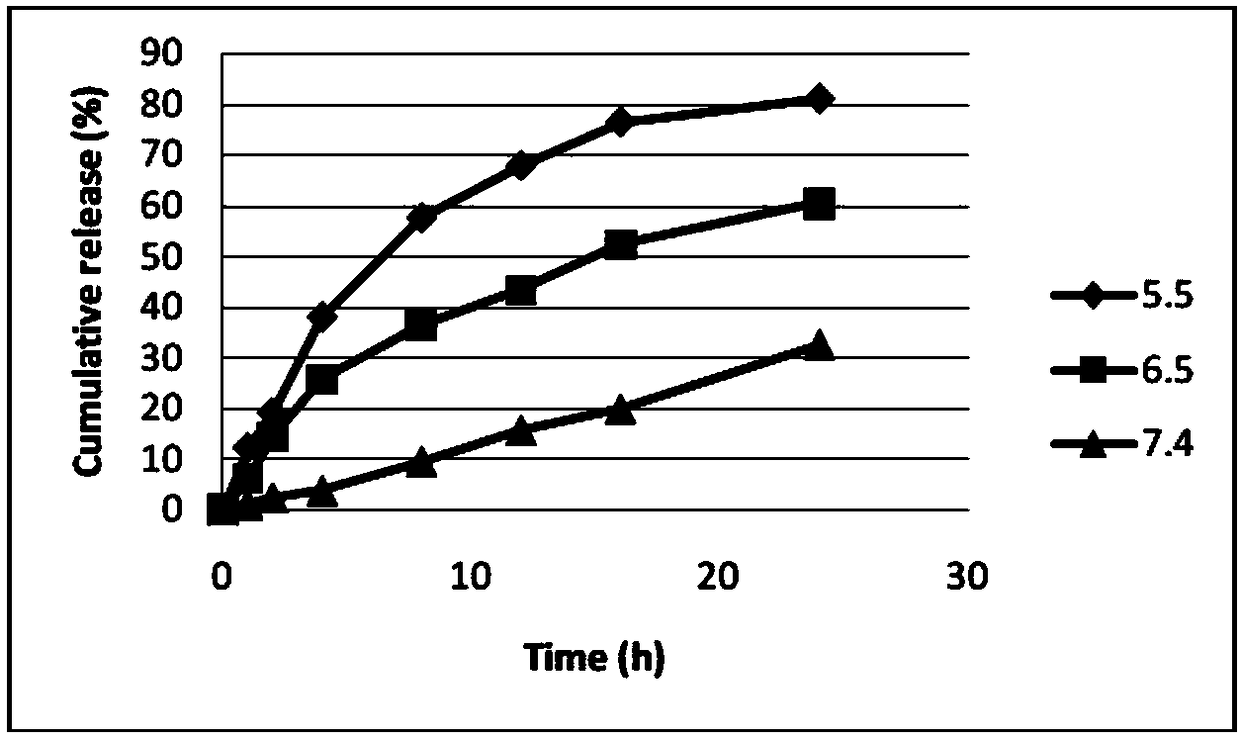
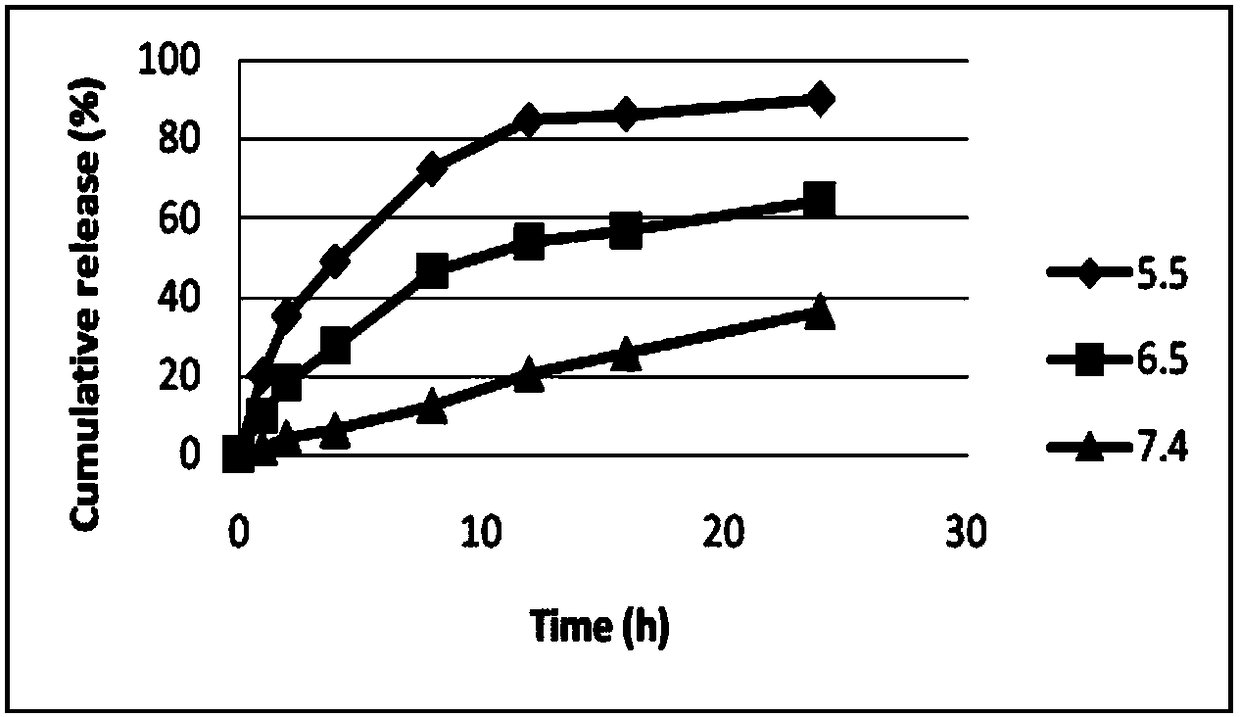
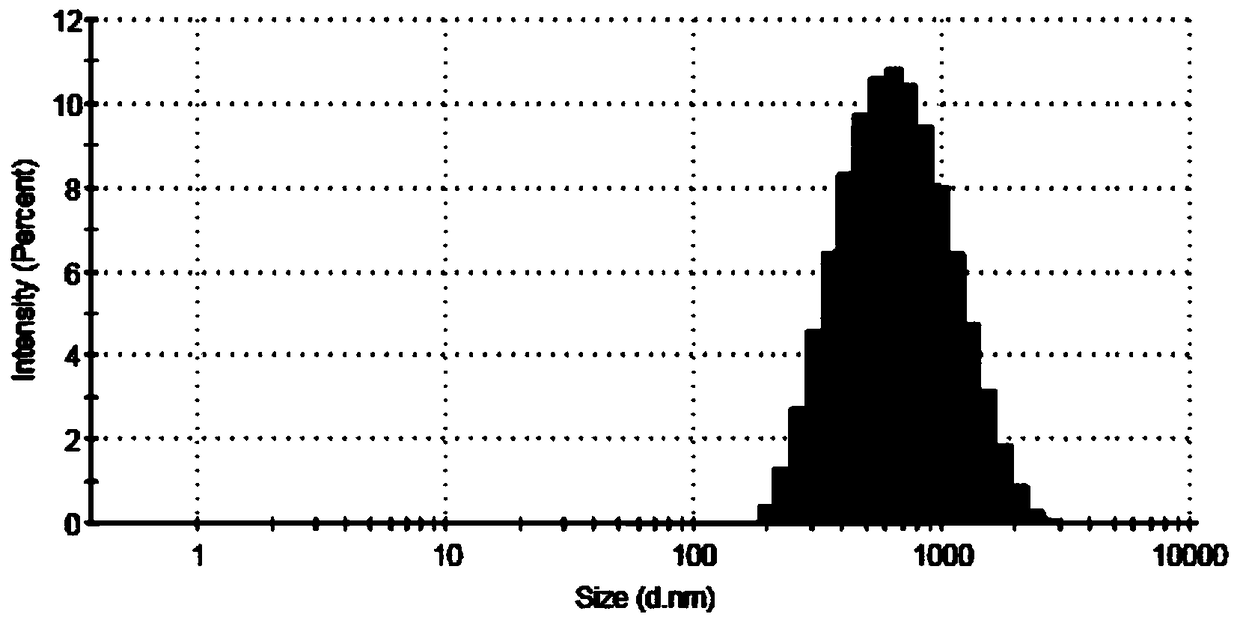
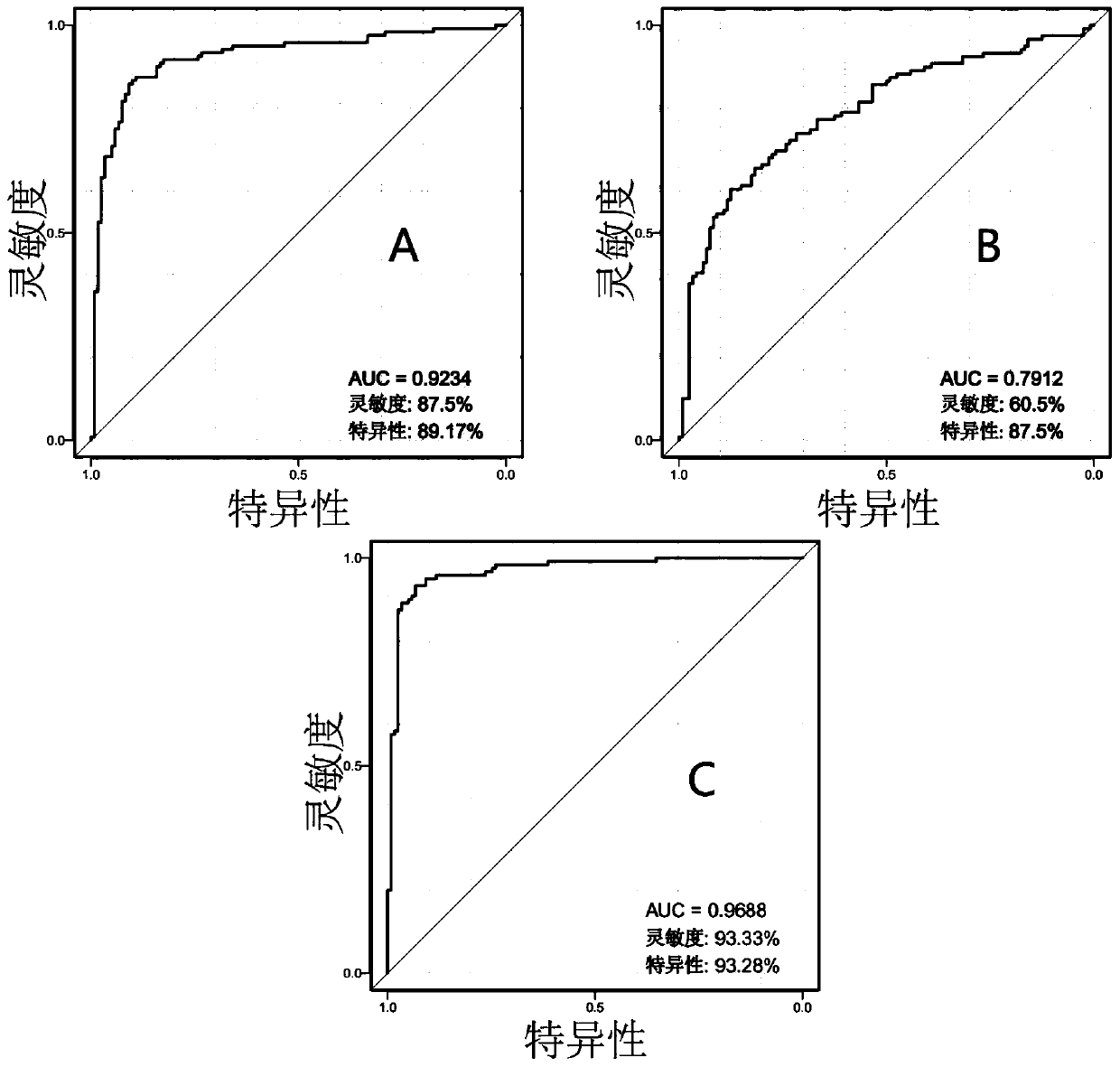
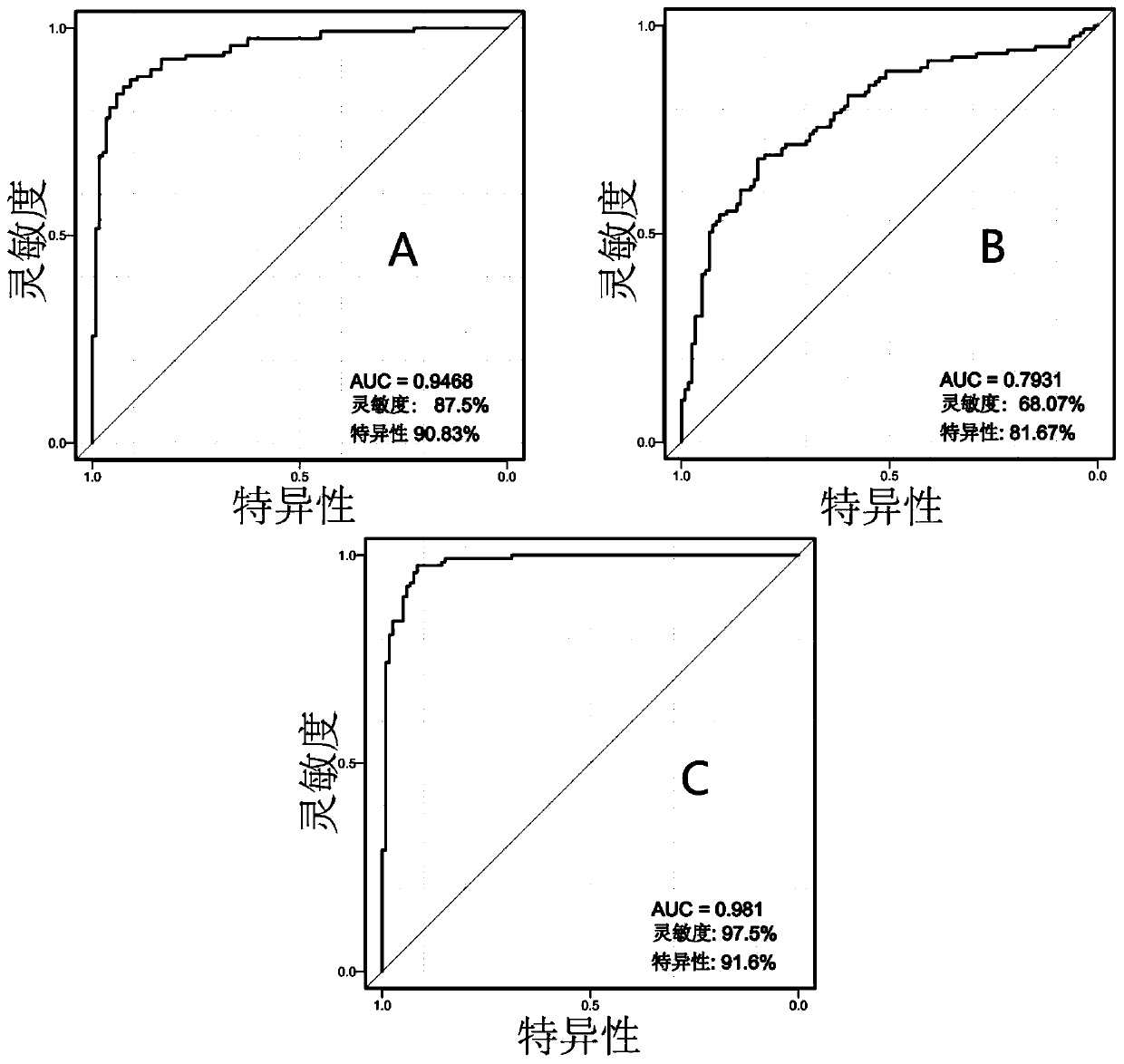
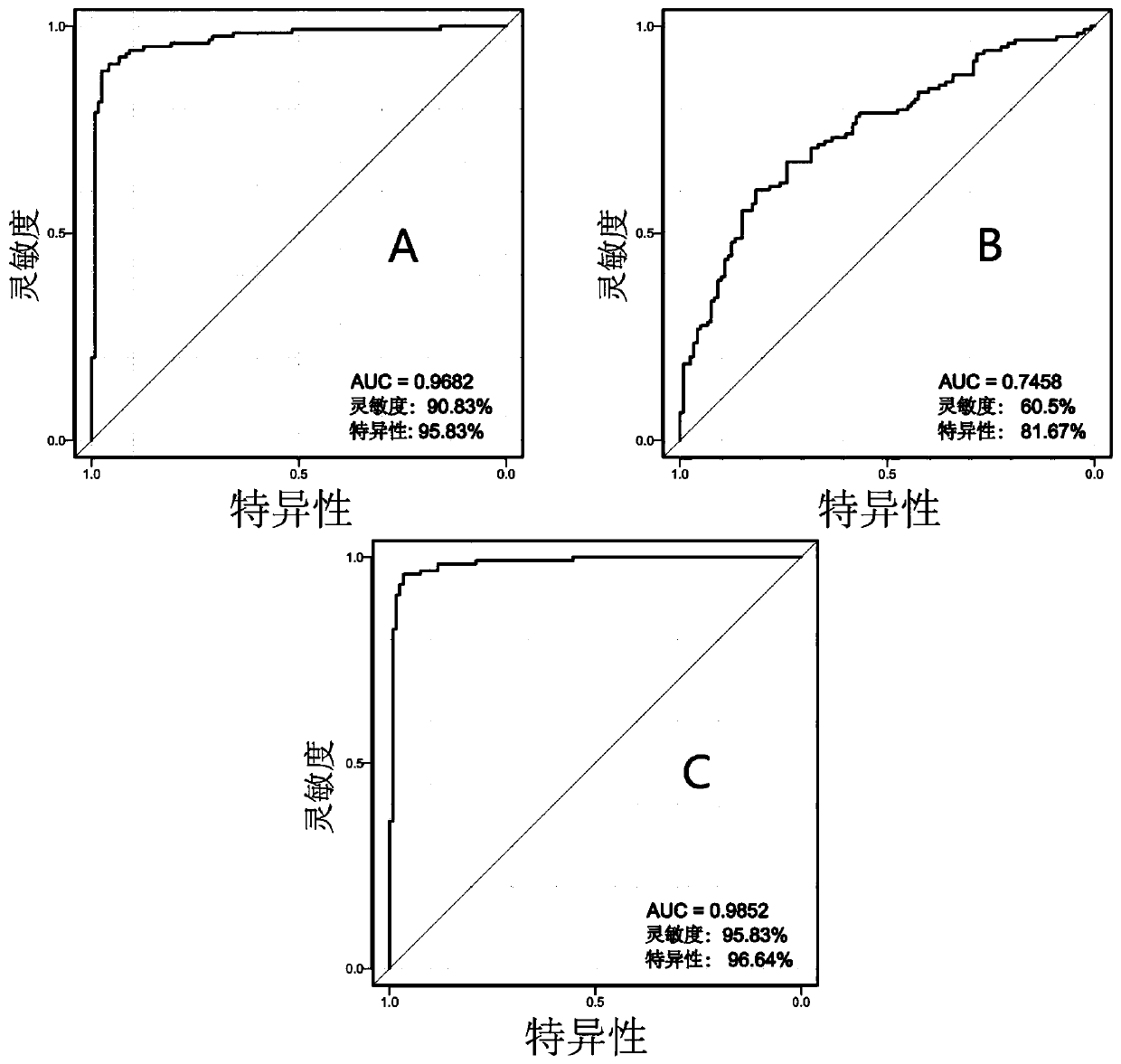
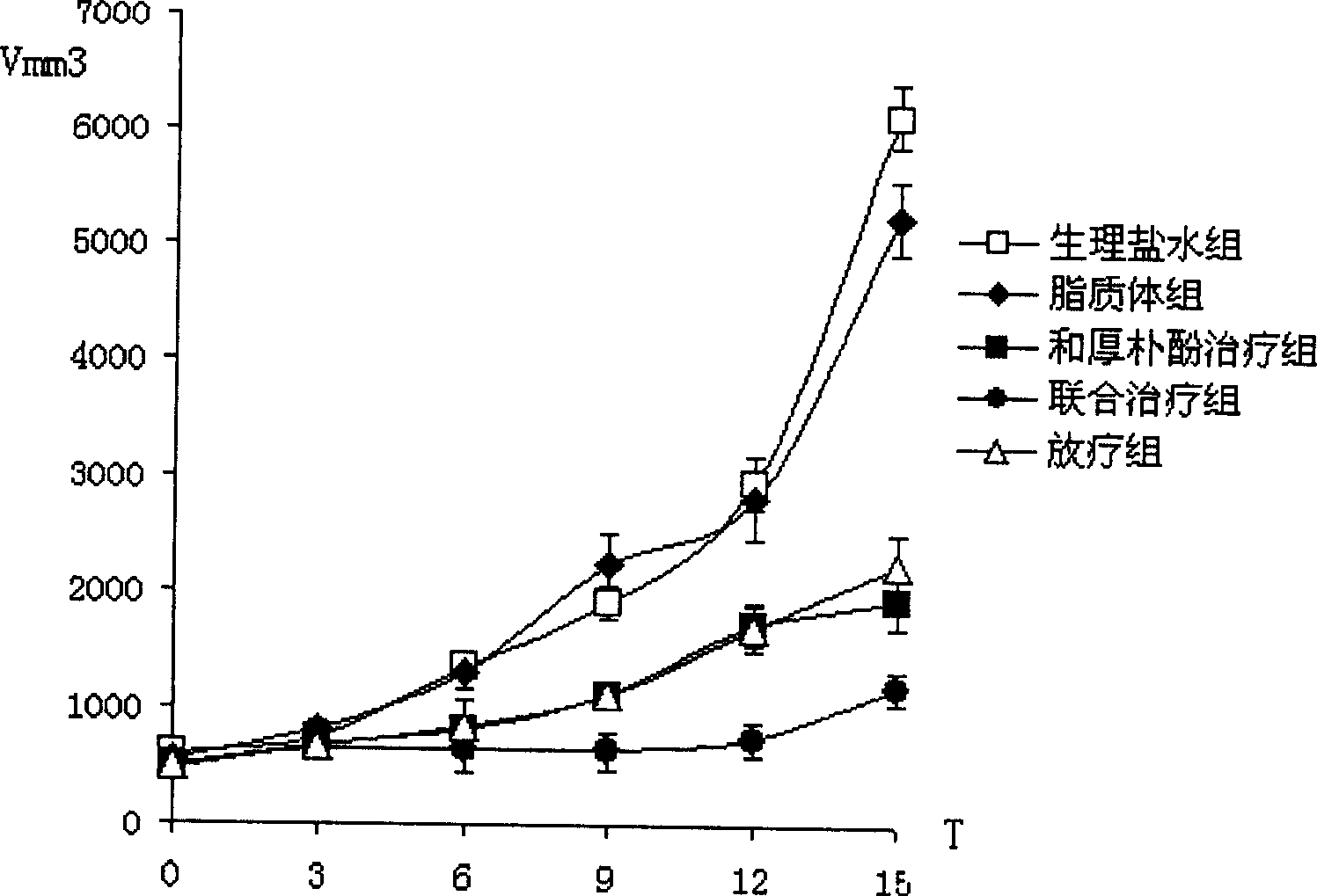
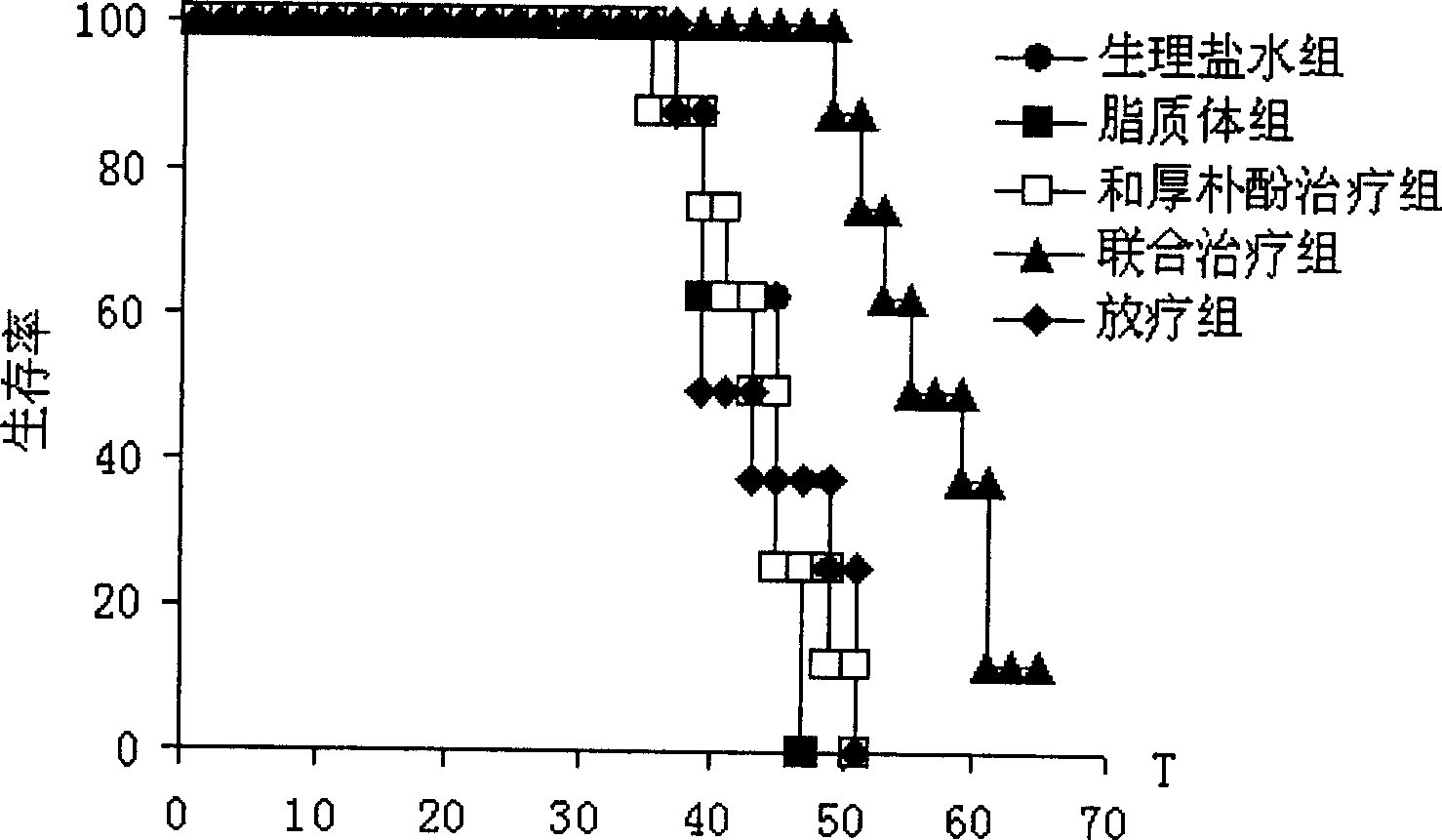
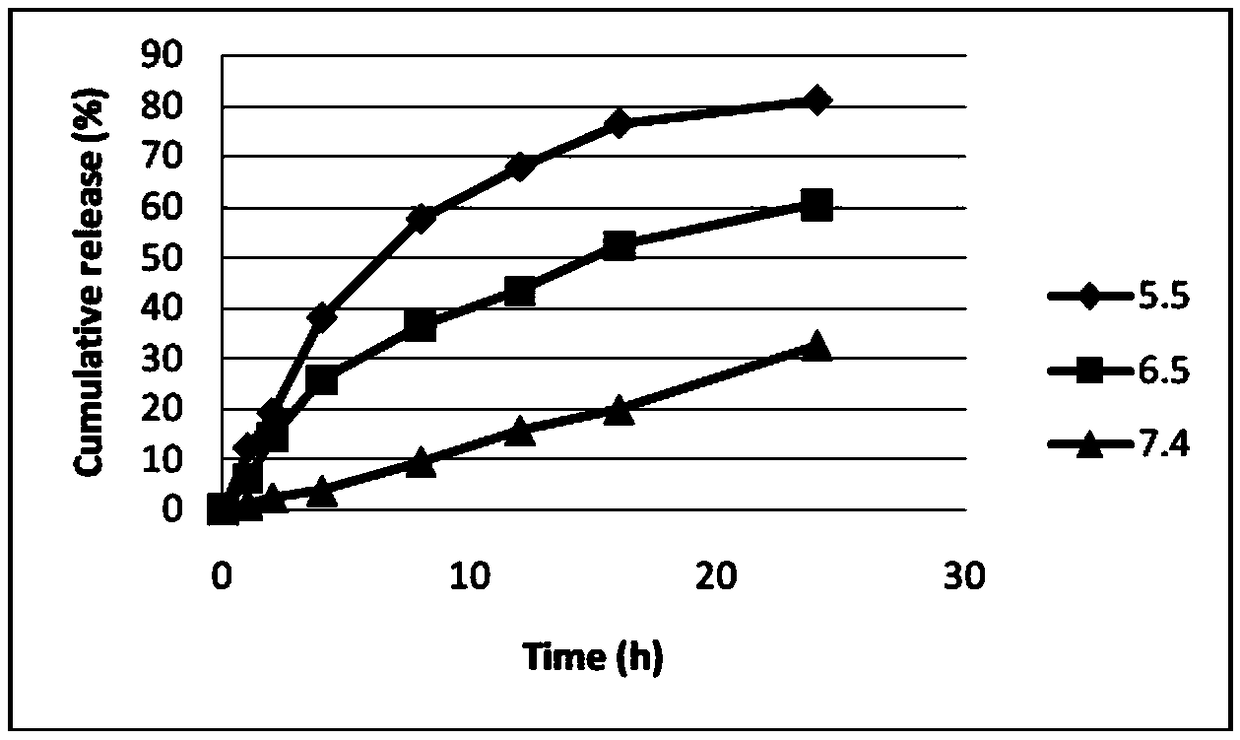
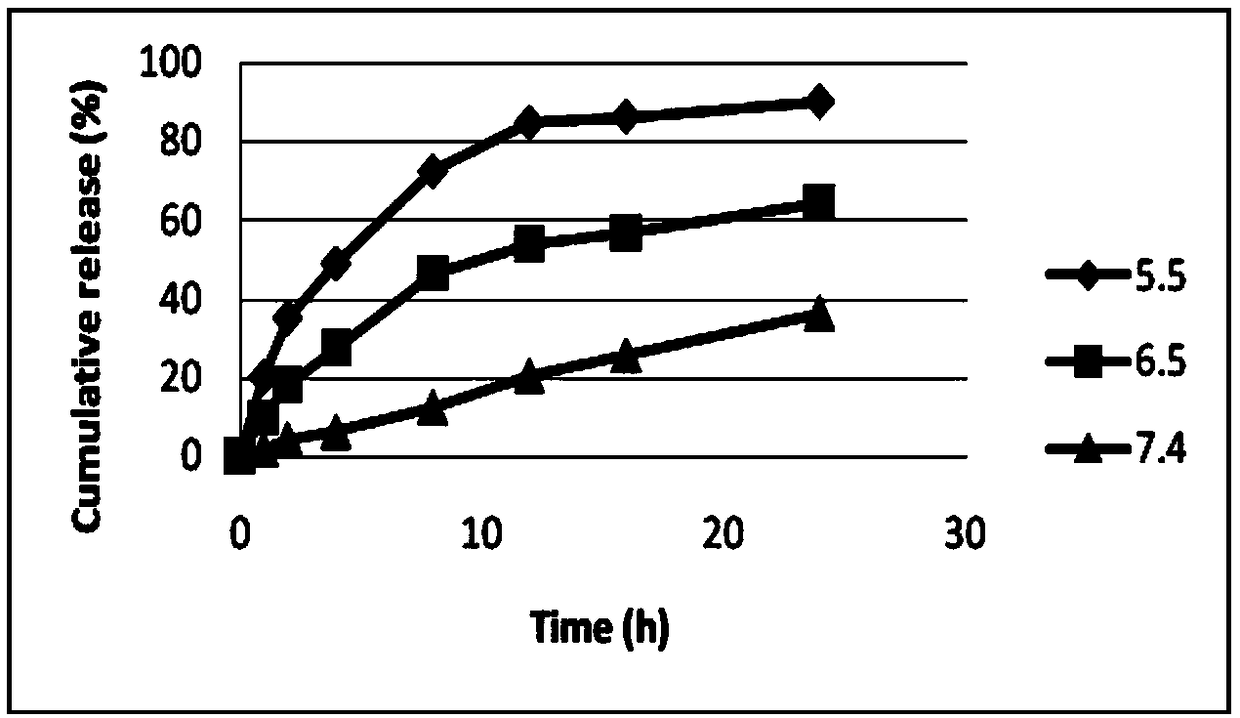
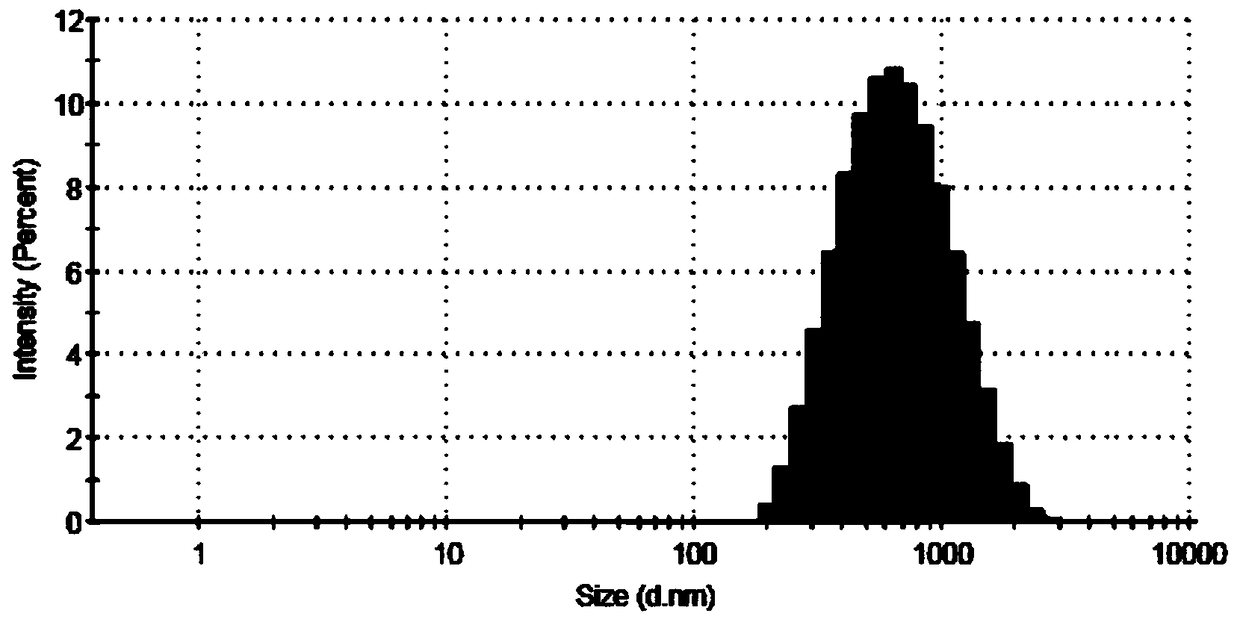
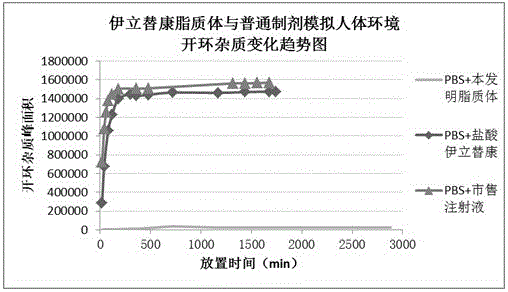
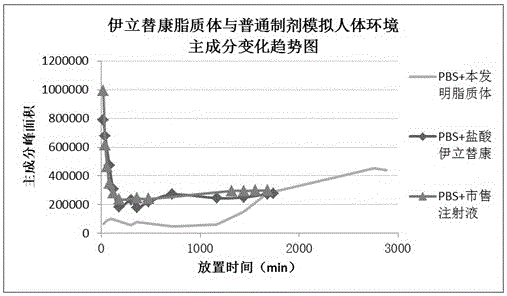
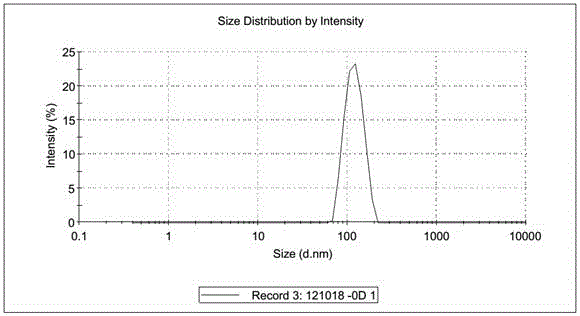
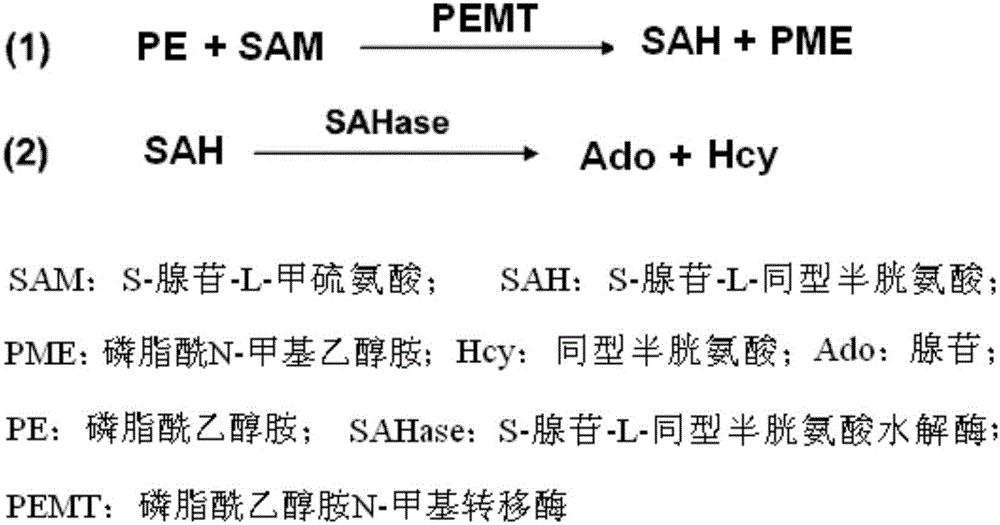
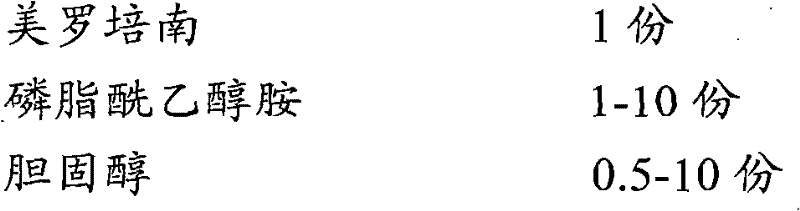Patents
Literature
35 results about "Phosphatidylethanol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphatidylethanols (PEth) are a group of phospholipids formed only in the presence of ethanol via the action of phospholipase D. Levels of phosphatidylethanols in blood are used as markers of previous alcohol consumption. For this purpose, PEth is more sensitive than carbohydrate deficient transferrin (CDT), urinary ethyl glucuronide (EtG) and ethyl sulfate (EtS).
Quercetin long-acting liposome powder for injection and its preparing method
InactiveCN1813677AProlong blood circulation timePromote absorptionOrganic active ingredientsPowder deliveryCholesterolPolyethylene glycol
The present invention relates to a quercetin long-acting liposome powder injection and its preparation method. Said powder injection is formed from quercetin, polyethylene glycol-ethanolamine phosphoglycerides, lecithin, cholesterol and excipient. Said invention also provides the concrete steps of its preparation method.
Owner:SICHUAN UNIV
Officinal magnolia phenol lipid frozen dried powder preparation and its use in preparing drug for cancers
ActiveCN1895237AImprove the efficiency of tumor suppressionBoost and/or modulate immunityPowder deliveryHydroxy compound active ingredientsOfficinalCholesterol
A freeze-dried powder of honokiol liposome for preparing the medicines to treat lung cancer and mammary cancer is proportionally prepared from honokiol, polyethanediol-phosphatidylethanolamine, lecithin and cholesterol. It has high synergistic and sensitizing action when it is applied in conjunction with chemicotherapeutic medicine.
Owner:CHENGDU JINRUI FOUND BIOTECH CO LTD
Propranolol hydrochloride lipidosome gel and preparation method thereof
ActiveCN103622903AImprove stabilityProlonged dosing timeOrganic active ingredientsAerosol deliveryWhole bodyCholesterol
The invention discloses a propranolol hydrochloride lipidosome gel, which is prepared by the following bulk drugs and auxiliary materials by weight percent: 0.012-0.075% of propranolol hydrochloride, 0.037-0.150% of phosphatidyl ethanolamine, 0.012-0.075% of cholesterol, 2.5-5% of triethanolamine, 1-2% of carbopol, and the balance of water. According to the propranolol hydrochloride lipidosome gel, during the preparation, the lipidosome is uniformly dispersed in the gel, so that the stability of the lipidosome is improved; the water-soluble gel carbopol has excellent biocompatibility, can be well adhered to skin and cannot stimulate skin; drug administration time is prolonged, the toxic and side effects on the whole body are reduced, the adaptability of patients is improved, and the propranolol hydrochloride lipidosome gel has excellent application prospect.
Owner:SHANDONG UNIV
Breathable anti-abrasion anti-electrostatic and waterproof fabric and preparing method thereof
InactiveCN107740197AStable and guaranteed waterproof levelBreathable and wear-resistantConjugated cellulose/protein artificial filamentsArtificial filament heat treatmentPolyesterEthanolamine synthesis
The invention relates to the field of fabric production, in particular to a breathable anti-abrasion anti-electrostatic and waterproof fabric and a preparing method thereof. The fabric is formed by blending cotton and linen fiber, viscosity fiber and anti-electrostatic polyester fiber; the fabric is prepared from, by weight, 30-40 parts of cotton and linen fiber, 35-40 parts of viscosity fiber, 30-40 parts of anti-electrostatic polyester fiber, 33-46 parts of polylactic acid, 25-38 parts of metasilicic acid trimethyl ester, 22-28 parts of dilauryl thiodipropionate, 21-25 parts of sodium dodecyl benzene sulfonate, 13-17 parts of phosphatidyl ethanolamine, 9-14 parts of potassium metabisulfite, 8-9 parts of cetyl trimethyl ammonium bromide, 8-9 parts of hydroxypropyl starches, 5-7 parts of nano silicon dioxide, 5-7 parts of sodium cetyl sulfate, 4-6 parts of linoleic acid, 3-4 parts of silane coupling agent, 3-4 parts of binding agent and 180 parts of deionized water.
Owner:JIANGSU AOYANG SHIJIA CLOTHING CO LTD
Long-circulation irinotecan lipidosome composition and preparation method thereof
InactiveCN103830182AHas a long cycle effectHigh tumor inhibition rateOrganic active ingredientsPowder deliverySide effectCholesterol
The invention belongs to the field of medicine preparations, which particularly relates to a long-circulation irinotecan lipidosome composition. The long-circulation irinotecan lipidosome composition is prepared from the following components in parts by weight: 1 part of irinotecan, 2 to 4 parts of hydrogenated soy phosphatidylcholine or distearoyl lecithin, 0.5 to 1.5 parts of cholesterol, 0.4 to 1.2 parts of phosphatidylethanolamine pegol and 0.0104 to 0.051 part of metal-chelator. The invention further discloses a preparation method of the long-circulation irinotecan lipidosome composition and an application of the long-circulation irinotecan lipidosome composition prepared into an irinotecan lipidosome composition injection. According to the irinotecan lipidosome composition and the preparation method thereof, the medicine loading ratio of the irinotecan is greatly improved and the encapsulation efficiency is greater than 99.5%, so that the toxic and side effect of the medicine which is not encapsulated can be obviously reduced; the content of relevant substances is lower than 0.5% and the limit of various toxic impurities is lower than 0.1%, so that the safety of the composition is obviously improved; and the composition is uniform in granularity distribution and good in stability.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Quercetin long-acting liposome powder for injection and its preparing method
InactiveCN100370968CGood water solubilityImprove stabilityOrganic active ingredientsPowder deliveryCholesterolPolyethylene glycol
Owner:SICHUAN UNIV
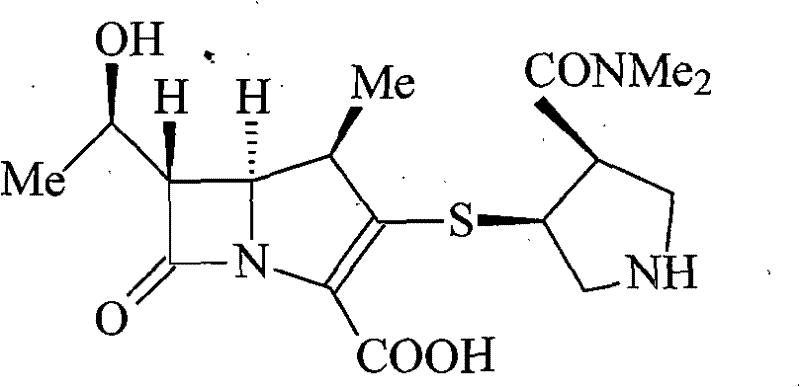
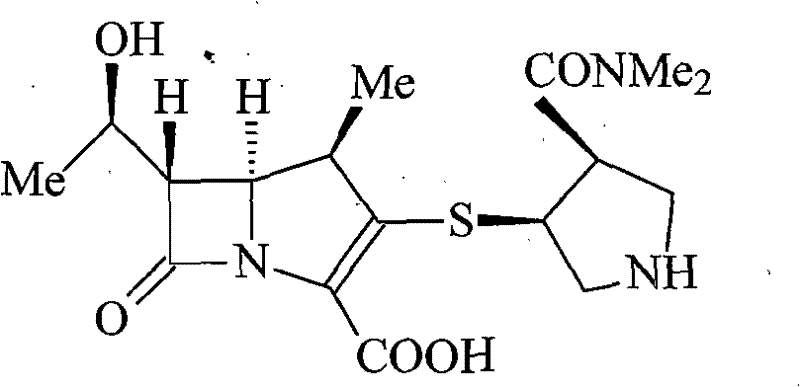
A kind of meropenem liposome injection
InactiveCN102258487AGood resolubilityImprove stabilityAntibacterial agentsOrganic active ingredientsSide effectAntioxidant
The invention discloses a meropenem liposome injection, which is characterized by mainly comprising the following components of: by weight, one part of meropenem, 2-8 parts of phosphatidylethanolamine, 1-5 parts of cholesterol, 0.2-1 part of polyether 188, 0.1-0.4 part of an antioxidant, and 5-10 parts of a supporting agent. The liposome injection prepared in the invention has good redissolving performance, good stability, high entrapment rate and little side-effect, can be used to raise the preparation product quality, minimize toxic and side effects and improve the bioavailability, and has remarkable curative effects.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
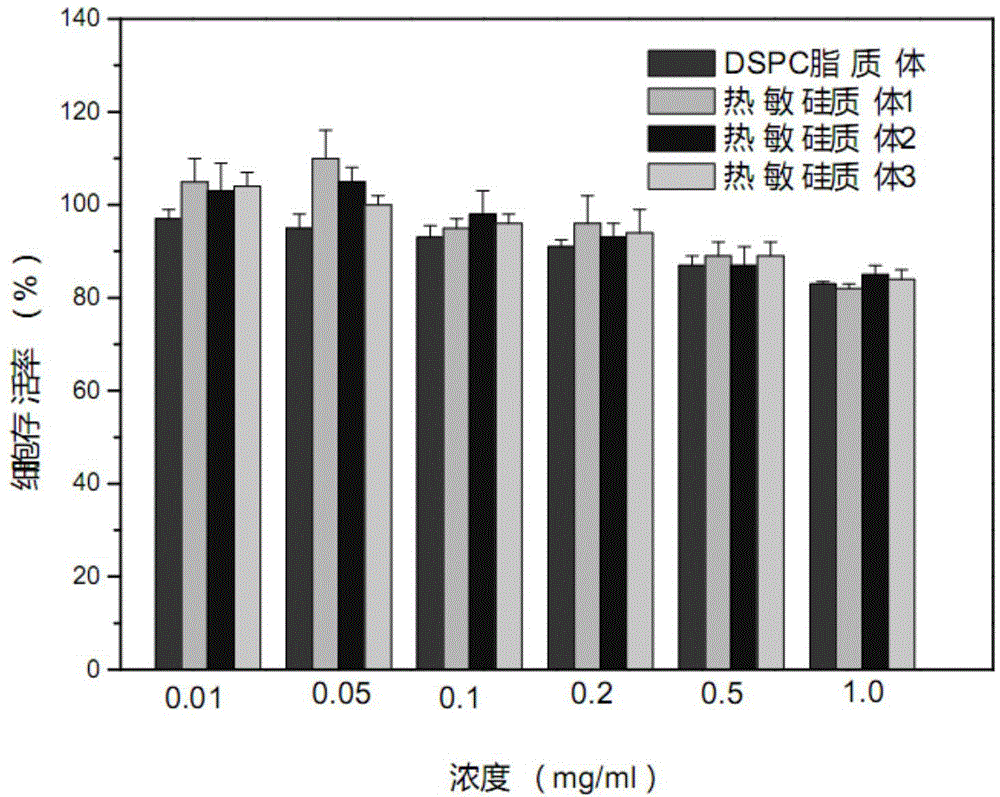
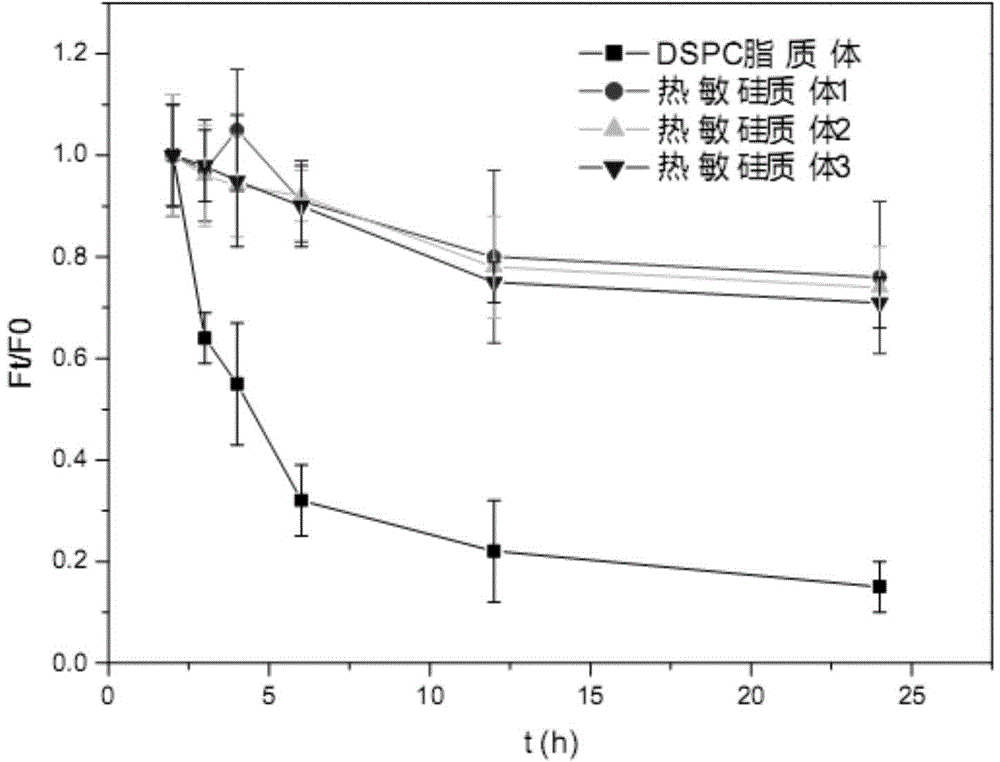
Thermosensitive silica body and preparing method and application thereof
InactiveCN105194678AImprove HIFU response performanceIncrease temperatureEnergy modified materialsPharmaceutical non-active ingredientsTreatment effectPolyethylene glycol
The invention relates to a thermosensitive silica body and a preparing method and application thereof. The thermosensitive silica body and high-intensity focused ultrasound beams (HIFU) are combined and used for controlling release of hydrophobic drugs. The thermosensitive silica body is prepared from, by weight, 41.90-62.90 parts of lipid formed by the silica body, 40.00-60.00 parts of dipalmitoyl phosphatidyl choline, 5.20-8.50 parts of palmitoyl phosphatidyl choline and 14.00-19.50 parts of polyethylene glycol 2000-dipalmitoyl phosphatidyl ethanolamine. The thermosensitive silica body is high in stability and biocompatibility and capable of fast releasing the hydrophobic antineoplastic drugs when combined with HIFU and shows a remarkable therapeutic effect.
Owner:BEIJING SAMSUNG TELECOM R&D CENT +2
Application of group of metabolic markers in early diagnosis of metabolic syndrome
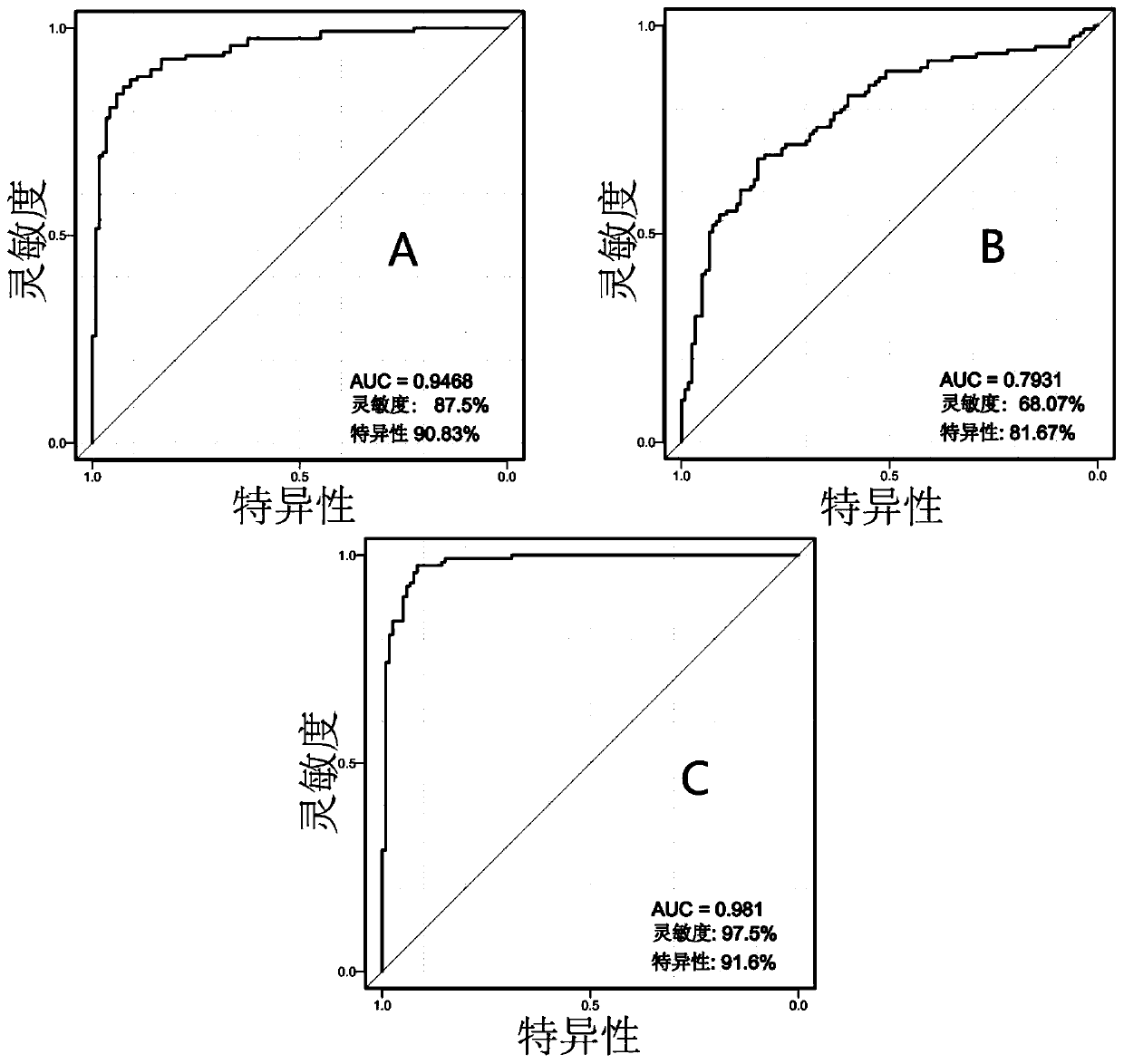
The invention discloses an application of a group of metabolic markers in early diagnosis of a metabolic syndrome. The metabolic markers at least comprise glutamine-leucine and phosphatidyl ethanolamine PE (18:2(9Z,12Z) / P-18:0), and further comprise tyrosine or phenylalanine. In a validation set of a specific embodiment, the accuracy degree for distinguishing a health volunteer and a metabolic syndrome patient through joint use of tyrosine, phosphatidyl ethanolamine PE (18:2(9Z,12Z) / P-18:0) and glutamine-leucine reaches 80.5%, and the accuracy degree for distinguishing the health volunteer andthe metabolic syndrome patient through joint use of phenylalanine, glutamine-leucine and phosphatidyl ethanolamine PE (18:2(9Z,12Z) / P-18:0)reaches 82.6%. Therefore, the abovementioned metabolic markers have high accuracy degree for diagnosis of the metabolic syndrome, can be prepared into a metabolic syndrome diagnosis kit and are used for early diagnosis of a healthy person suffering from the metabolic syndrome.
Owner:CHINA PHARM UNIV
Method for quickly modifying avidin on interface based on lipidosome
InactiveCN106754860AGood biocompatibilityReduce distractionsOn/in inorganic carrierPhospholipidNitrogen gas
The invention relates to a method for quickly modifying avidin on an interface based on lipidosome. The method comprises the steps of mixing phospholipid and biotinylated phosphatidyl ethanol, adding chloroform for ultrasonic stirring, drying under nitrogen, and vacuum drying; adding a neutral solution, matching films with different bore diameters for a squeezer, and squeezing for multiple times so as to form lipidosomes with different sizes; after cleaning a glass slide, soaking in a hydrofluoric acid solution with the concentration being 1 percent for dozens of seconds, then washing with pure water, and drying under the nitrogen; putting the glass slide into a clean utensil, dropwise adding a certain amount of synthetic lipidosome, incubating for several minutes at the temperature higher than the phase-transition temperature, and using super-pure water for cleaning excessive lipidosome; later, adding the avidin for incubating for several minutes, using the super-pure water or solution to clean excessive avidin, and obtaining a glass interface with the avidin modified on the surface. The method is simple, convenient and quick in modification steps, uniform in modification, and complete in sealing, and since silanization reagents are not introduced during a modification process, the background interference is less. The prepared interface can meet the requirement on single molecule enzymology research.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
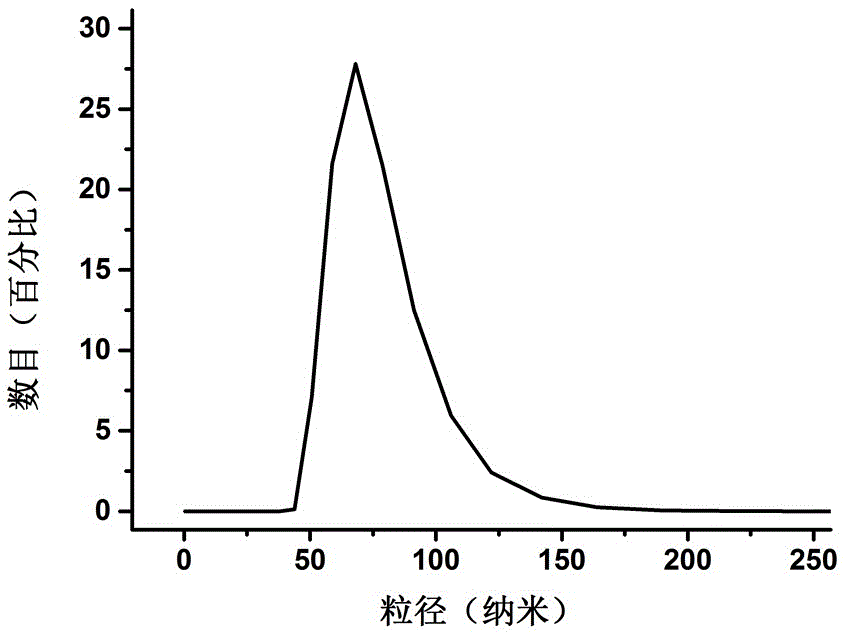
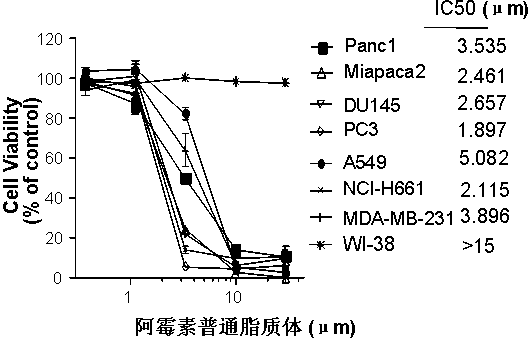
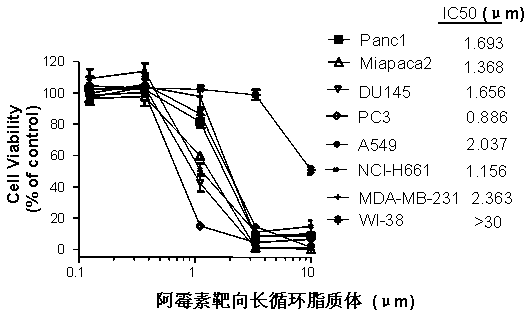
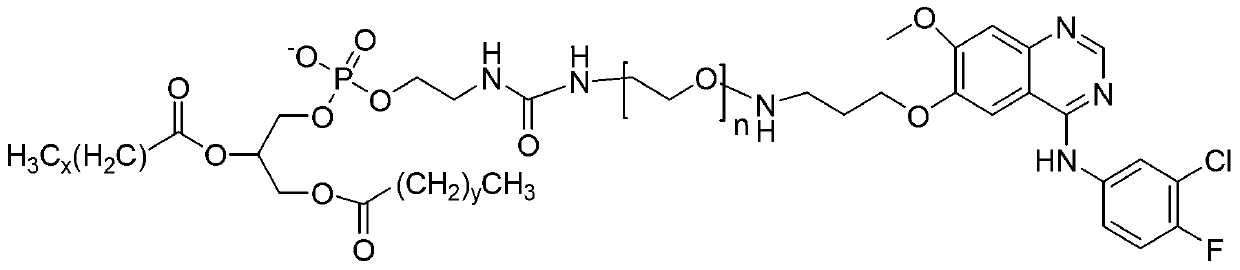
Polyethylene glycol-modified phospholipid derivative taking anilino-quinazoline as targeting ligand and preparation method thereof
InactiveCN102649841BPharmaceutical non-active ingredientsAntineoplastic agentsSulfonyl chlorideSide effect
The invention provides a polyethylene glycol-modified phospholipid derivative taking a 4-substituted anilino-quinazoline group as a targeting ligand and a preparation method thereof. The method comprises the following steps of: performing condensation with para-methylbenzene sulfonyl chloride, substitution with phthalimide potassium salt and hydrolysis with a hydrazine hydrate by taking polyethylene glycol (PEG) as a starting raw material to obtain diamine PEG; and reacting with targeting ligands, i.e., N-(3-chlorin-4-fluorin)-6-(3-chloropropyl)-7-methoxylquinazoline-4-amine and distearoylphosphatidylethanolamine (DSPE) to respectively obtain 4-anilino-quinazoline group-PEG-DSPE. A liposome preparation of the 4-anilino-quinazoline group-PEG-DSPE can be used for realizing target administration of tumor cells and reducing the toxic and side effects of a tumor medicament; and the pharmacodynamic structure of gefitinib can give play to dual medicament effects together with other liposome-coated antitumor medicaments, so that the tumor treatment effect is enhanced greatly.
Owner:SOUTHEAST UNIV
SPIO labeled pH sensitive drug-carrying liposome and preparation method thereof
ActiveCN108542884AHigh pH sensitivityGood superparamagneticOrganic active ingredientsHeavy metal active ingredientsFreeze thawingSuperparamagnetic iron oxide nanoparticles
The invention discloses an SPIO labeled pH sensitive drug-carrying liposome which is prepared from the following components in parts by mass: 1-10 parts of bulk drugs, 1-50 parts of phosphatidyl ethanolamine, 1-30 parts of cholesterol, 1-30 parts of linoleic acid, 0-10 parts of carboxymethyl chitosan, and 0-10 parts of superparamagnetic iron oxide nanoparticles. The invention further discloses a preparation method of the SPIO labeled pH sensitive drug-carrying liposome. The preparation method comprises the following steps: S1, placing phosphatidyl ethanolamine, cholesterol and linoleic acid ina flask, adding chloroform for dissolving, adding a water soluble drug solution for ultrasonic treatment to form a W / O emulsion; S2, evaporating to a gel state under the pressure reduction condition;adding a buffering solution, and continuously evaporating to form an aqueous suspension; S3, adding the superparamagnetic iron oxide nanoparticles and performing freeze thawing on a liposome suspension for three times; and centrifugally removing the superparamagnetic iron oxide nanoparticles and the water soluble drug not in the aqueous suspension; and S4, adding a carboxymethyl chitosan solutionfor hydration.
Owner:重庆市人民医院
Application of a group of metabolic markers in early diagnosis of metabolic syndrome
The invention discloses an application of a group of metabolic markers in early diagnosis of a metabolic syndrome. The metabolic markers at least comprise glutamine-leucine and phosphatidyl ethanolamine PE (18:2(9Z,12Z) / P-18:0), and further comprise tyrosine or phenylalanine. In a validation set of a specific embodiment, the accuracy degree for distinguishing a health volunteer and a metabolic syndrome patient through joint use of tyrosine, phosphatidyl ethanolamine PE (18:2(9Z,12Z) / P-18:0) and glutamine-leucine reaches 80.5%, and the accuracy degree for distinguishing the health volunteer andthe metabolic syndrome patient through joint use of phenylalanine, glutamine-leucine and phosphatidyl ethanolamine PE (18:2(9Z,12Z) / P-18:0)reaches 82.6%. Therefore, the abovementioned metabolic markers have high accuracy degree for diagnosis of the metabolic syndrome, can be prepared into a metabolic syndrome diagnosis kit and are used for early diagnosis of a healthy person suffering from the metabolic syndrome.
Owner:CHINA PHARM UNIV
Officinal magnolia phenol lipid frozen dried powder preparation and its use in preparing drug for cancers
ActiveCN1895237BImprove solubilitySimple preparation processPowder deliveryHydroxy compound active ingredientsOfficinalCholesterol
A freeze-dried powder of honokiol liposome for preparing the medicines to treat lung cancer and mammary cancer is proportionally prepared from honokiol, polyethanediol-phosphatidylethanolamine, lecithin and cholesterol. It has high synergistic and sensitizing action when it is applied in conjunction with chemicotherapeutic medicine.
Owner:CHENGDU JINRUI FOUND BIOTECH CO LTD
Long-acting lyophilized powder injection preparation of cisatracurium besilate
InactiveCN106727366AGood storage stabilityExtended storage timeOrganic active ingredientsPowder deliverySide effectAntioxidant
The invention relates to a long-acting lyophilized powder injection preparation of cisatracurium besilate. The long-acting lyophilized powder injection preparation of the cisatracurium besilate is prepared from the following components in parts by weight: 3-10 parts of cisatracurium besilate, 10-40 parts of polyethylene glycol-phosphatidyl ethanolamine, 10-30 parts of soya bean lecithin, 5-15 parts of sodium deoxycholate, 0.15-0.50 part of an antioxidant, 0.5-1 part of polyvinylpyrrolidone and 5-25 parts of a frozen-dried supporting agent. The preparation can be used for intravenous administration, the problem of the mass stability is effectively solved, the pharmacodynamic action time and the bioavailability of the preparation are improved, and meanwhile, the toxic or side effect is also relatively reduced.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
A kind of spio traced pH-sensitive drug-loaded liposome and preparation method thereof
ActiveCN108542884BGood pH sensitivityGood superparamagneticOrganic active ingredientsHeavy metal active ingredientsFreeze thawingSuperparamagnetic iron oxide nanoparticles
The invention discloses an SPIO labeled pH sensitive drug-carrying liposome which is prepared from the following components in parts by mass: 1-10 parts of bulk drugs, 1-50 parts of phosphatidyl ethanolamine, 1-30 parts of cholesterol, 1-30 parts of linoleic acid, 0-10 parts of carboxymethyl chitosan, and 0-10 parts of superparamagnetic iron oxide nanoparticles. The invention further discloses a preparation method of the SPIO labeled pH sensitive drug-carrying liposome. The preparation method comprises the following steps: S1, placing phosphatidyl ethanolamine, cholesterol and linoleic acid ina flask, adding chloroform for dissolving, adding a water soluble drug solution for ultrasonic treatment to form a W / O emulsion; S2, evaporating to a gel state under the pressure reduction condition;adding a buffering solution, and continuously evaporating to form an aqueous suspension; S3, adding the superparamagnetic iron oxide nanoparticles and performing freeze thawing on a liposome suspension for three times; and centrifugally removing the superparamagnetic iron oxide nanoparticles and the water soluble drug not in the aqueous suspension; and S4, adding a carboxymethyl chitosan solutionfor hydration.
Owner:重庆市人民医院
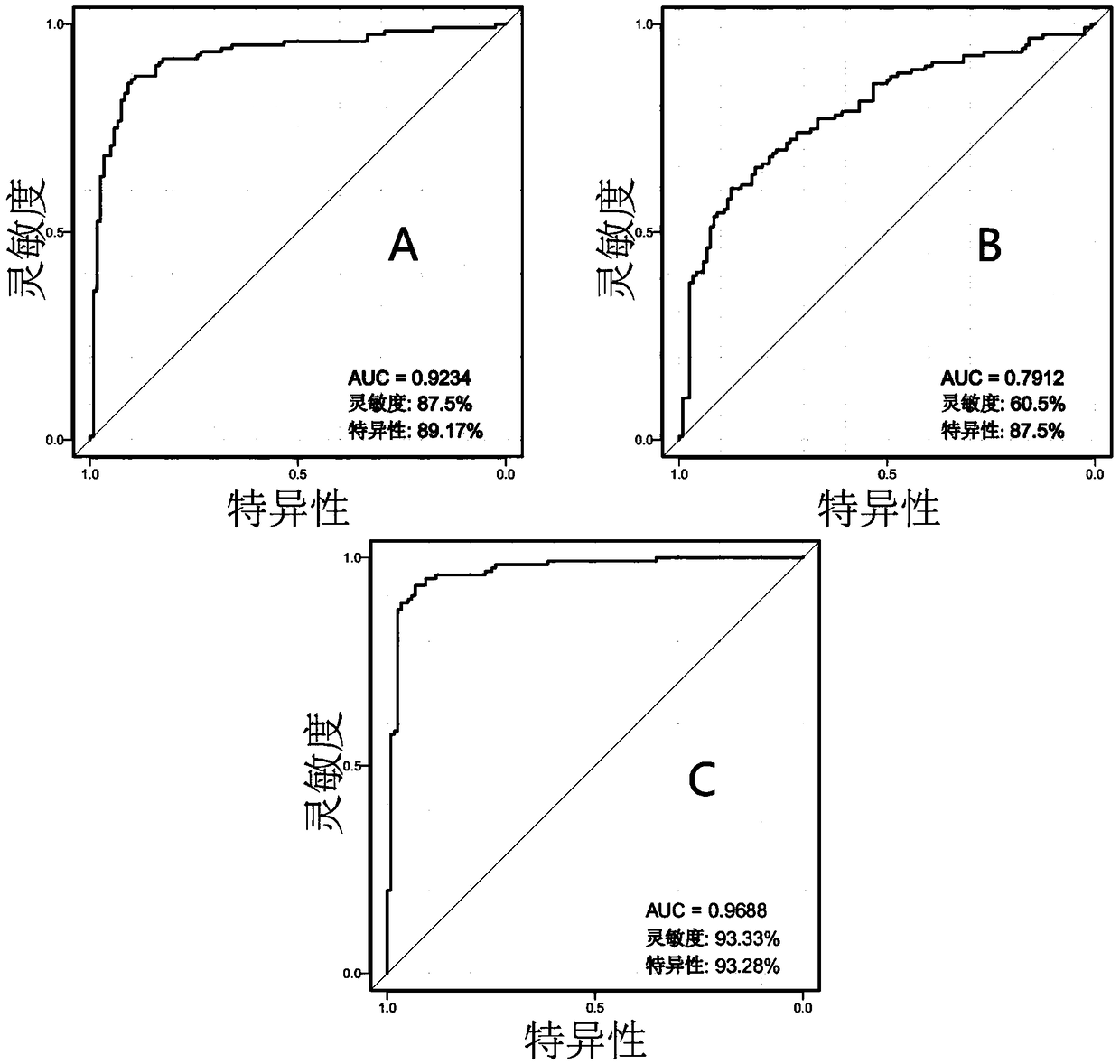
Application of p-hydroxycinnamic acid or phosphatidylethanolamine in early diagnosis of colorectal cancer
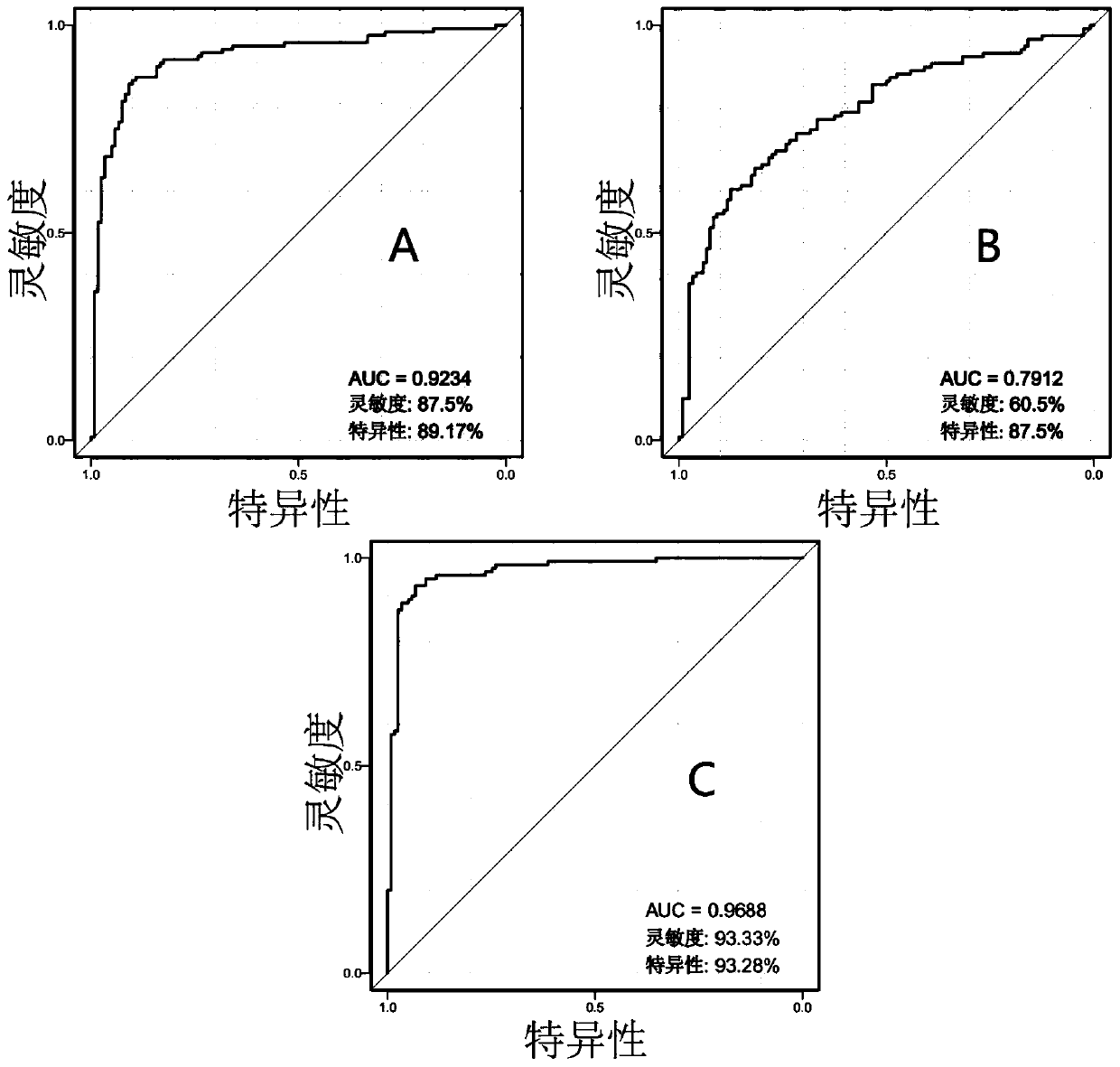
The invention discloses an application of hydroxycinnamic acid or phosphatidylethanolamine in early diagnosis of colorectal cancer. In a validation set of specific embodiments, the accuracy for distinguishing healthy subjects and colorectal cancer patients by taking hydroxycinnamic acid or phosphatidylethanolamine PE (18:2 (9Z,12z) / P-18:0) as a marker can reach 85.4% and 98.5%, the accuracy for distinguishing metabolic syndrome patients and colorectal cancer patients by taking hydroxycinnamic acid or phosphatidylethanolamine PE (18:2 (9Z,12z) / P-18:0) as the marker can reach 92.3% and 99.0%. Ascan be seen, hydroxycinnamic acid or phosphatidylethanolamine PE (18:2 (9Z,12z) / P-18:0) has a high diagnosis accuracy for colorectal cancer, can be prepared to a diagnosis kit for colorectal cancer and be used for early diagnosis of colorectal cancer in healthy people or metabolic syndrome patients.
Owner:CHINA PHARM UNIV
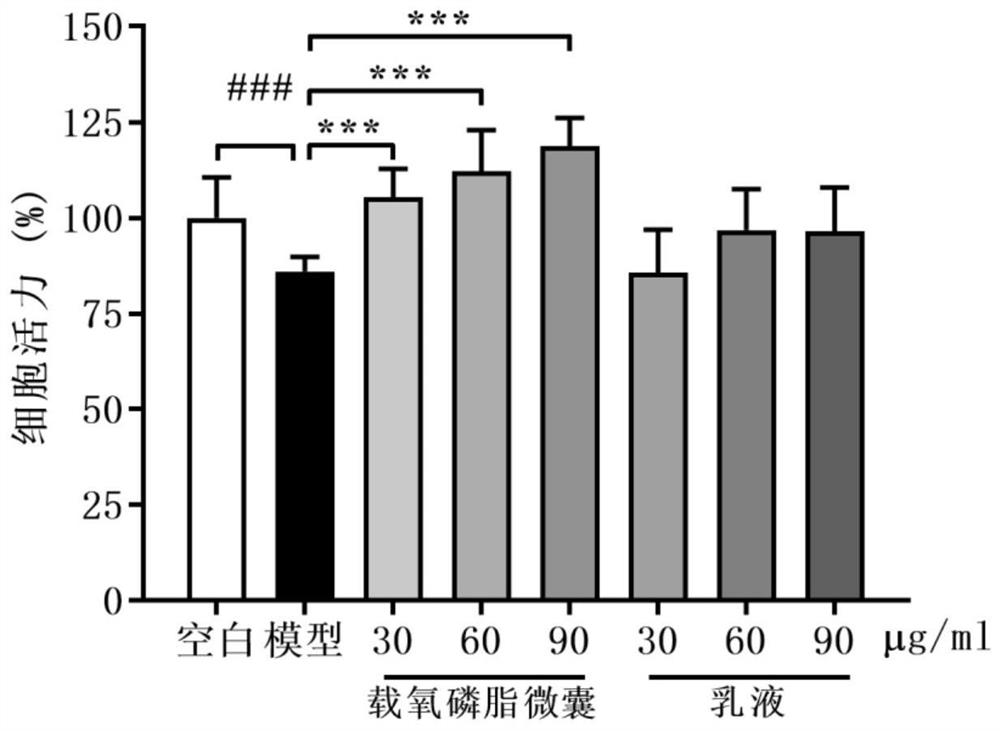
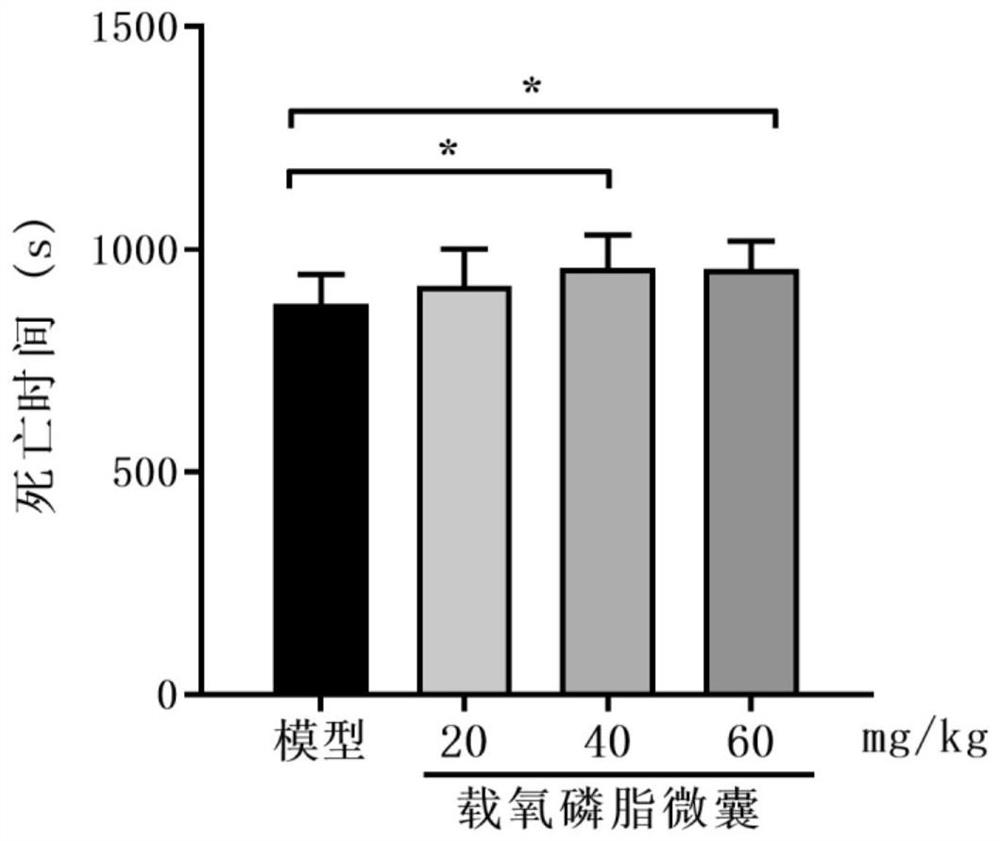
Oxygen-carrying phospholipid micro-capsule, preparation method thereof and application of oxygen-carrying phospholipid micro-capsule in preparation of anti-hypoxic drugs
PendingCN114288263AThe preparation method is simple and controllableEasy to implement transformationInorganic active ingredientsPharmaceutical non-active ingredientsFreeze-dryingPolyethylene glycol
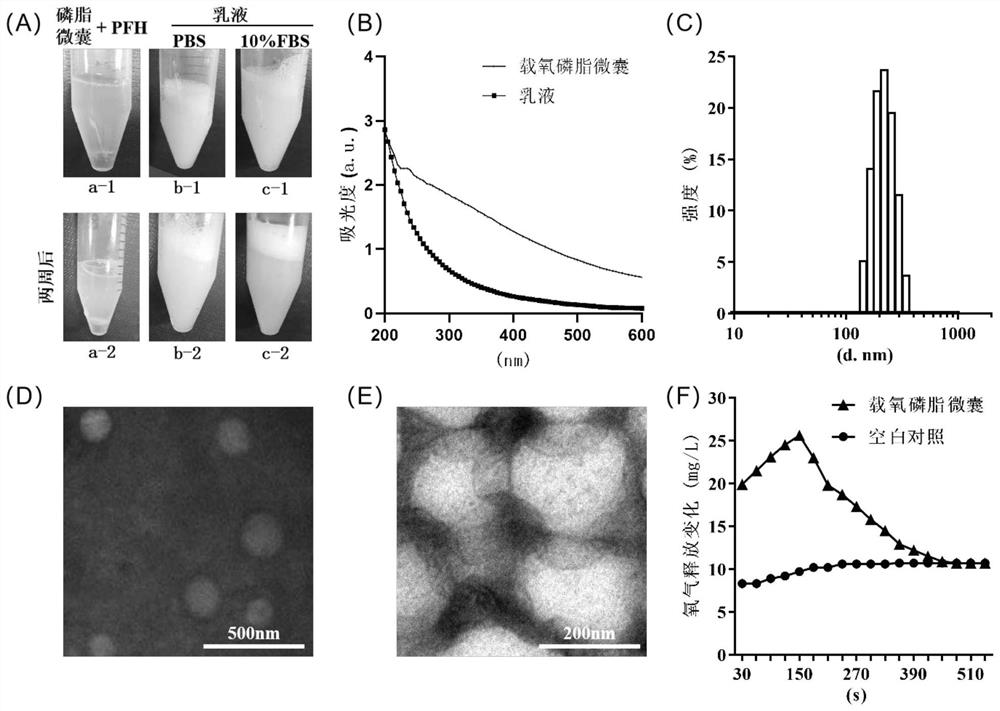
The invention discloses an oxygen-carrying phospholipid microcapsule, a preparation method thereof and application of the oxygen-carrying phospholipid microcapsule in preparation of an anti-hypoxia drug, and the preparation method comprises the following steps: adding myristoyl phosphatidylcholine, dipalmitoyl phosphatidylcholine, dipalmitoyl phosphatidylethanole-polyethylene glycol 2000-triphenyl phosphate and an organic solvent into a container, and carrying out ultrasonic treatment to obtain a clear solution; performing reduced pressure evaporation to remove the organic solvent, and adhering a layer of uniform and transparent faint yellow film on the inner wall of the container; carrying out vacuum drying, adding ultrapure water, hydrating in an ice bath to obtain phospholipid microcapsules, and putting the phospholipid microcapsules in the ice bath; adding perfluorohexane to obtain a mixed system, and performing ultrasonic dispersion until the mixed system is not layered to obtain an emulsion; and adding a freeze-drying protective agent, freeze-drying, storing at 4 DEG C, and redissolving and oxygenating before use to obtain the oxygen-carrying phospholipid microcapsule. The preparation method is simple and controllable, and raw materials and auxiliary materials are easy to obtain; the prepared oxygen-carrying phospholipid micro-capsule is uniform in particle size and good in stability and biocompatibility, oxygen can be supplemented to an organism in time, and the effect of treating diseases is achieved.
Owner:TIANJIN UNIV
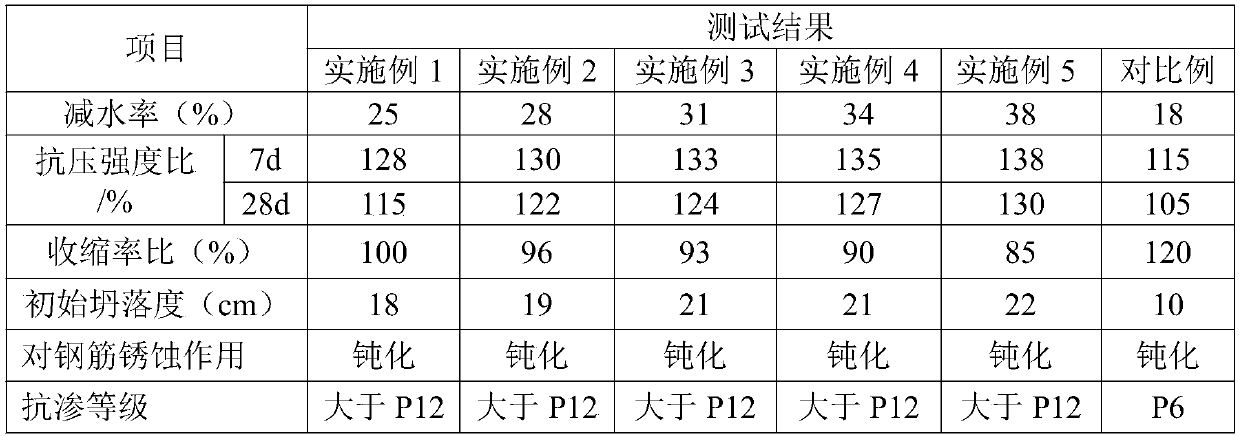
Concrete additive and preparation method thereof
InactiveCN109250948ASimple manufacturing methodRaw materials are easy to getPentaerythritolPolyphenyl ether
The invention provides a concrete additive which comprises the following components in parts by weight: 5-10 parts of polyurethane modified fly ash, 20-30 parts of indolyl sulfo diphenyl ketone polyphenyl ether, 10-15 parts of MPEG-2000-DSPE, 10-15 parts of pentaerythritol and 10-15 parts of deionized water. The invention also discloses a preparation method of the concrete additive. The preparation method of the concrete additive disclosed by the invention is simple and feasible, the raw materials are easy to obtain, the preparation cost is low, the dependence on equipment is small, and the method is suitable for large-scale production; the prepared concrete additive has various functions, is more obvious in improvement effect on the strength, fluidity, durability and impermeability of concrete, and has the advantages of being less in using amount and more excellent in comprehensive performance.
Owner:魏菊宁
Application of hydroxycinnamic acid or phosphatidylethanolamine in early diagnosis of colorectal cancer
The invention discloses an application of hydroxycinnamic acid or phosphatidylethanolamine in early diagnosis of colorectal cancer. In a validation set of specific embodiments, the accuracy for distinguishing healthy subjects and colorectal cancer patients by taking hydroxycinnamic acid or phosphatidylethanolamine PE (18:2 (9Z,12z) / P-18:0) as a marker can reach 85.4% and 98.5%, the accuracy for distinguishing metabolic syndrome patients and colorectal cancer patients by taking hydroxycinnamic acid or phosphatidylethanolamine PE (18:2 (9Z,12z) / P-18:0) as the marker can reach 92.3% and 99.0%. Ascan be seen, hydroxycinnamic acid or phosphatidylethanolamine PE (18:2 (9Z,12z) / P-18:0) has a high diagnosis accuracy for colorectal cancer, can be prepared to a diagnosis kit for colorectal cancer and be used for early diagnosis of colorectal cancer in healthy people or metabolic syndrome patients.
Owner:CHINA PHARM UNIV
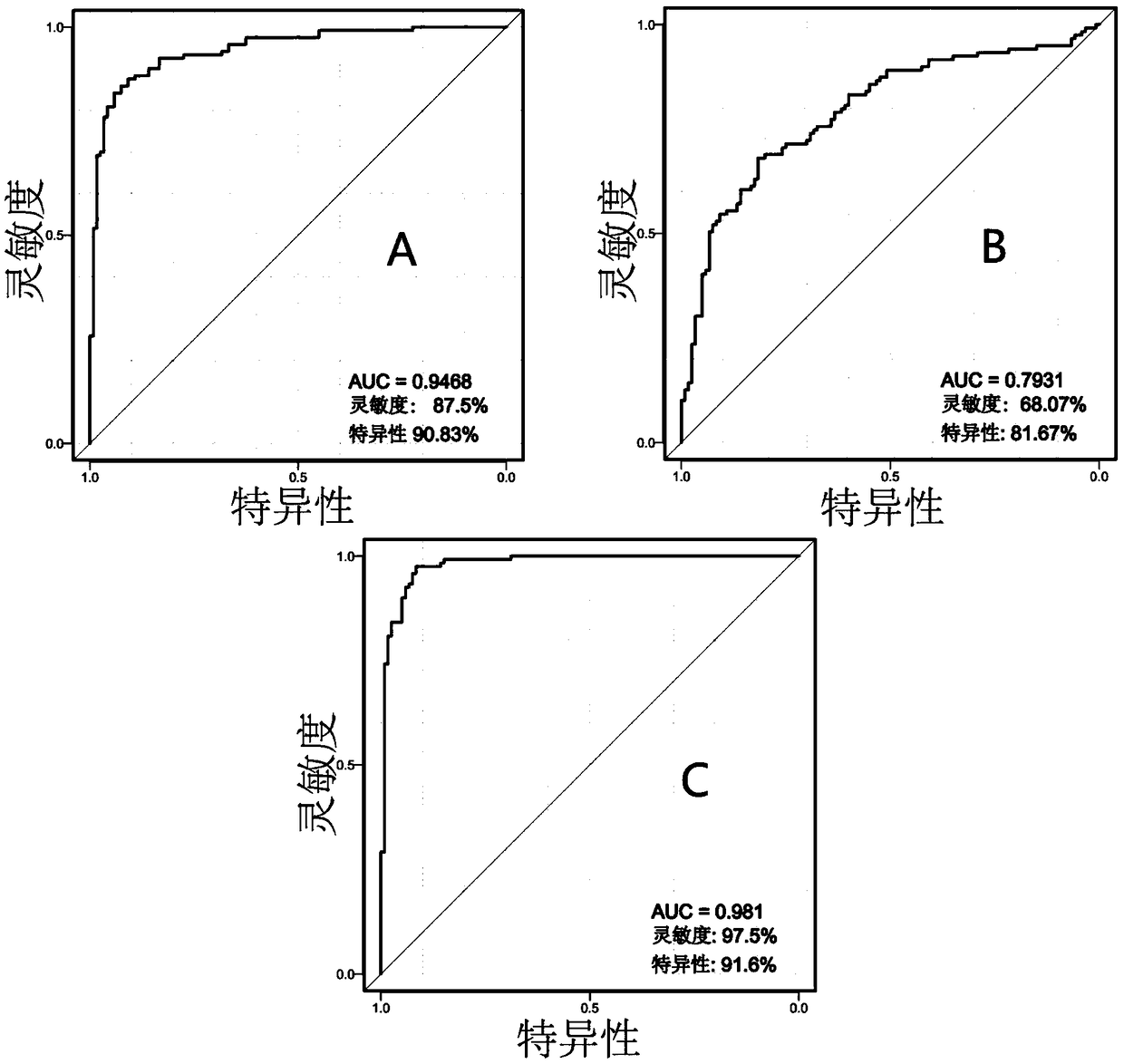
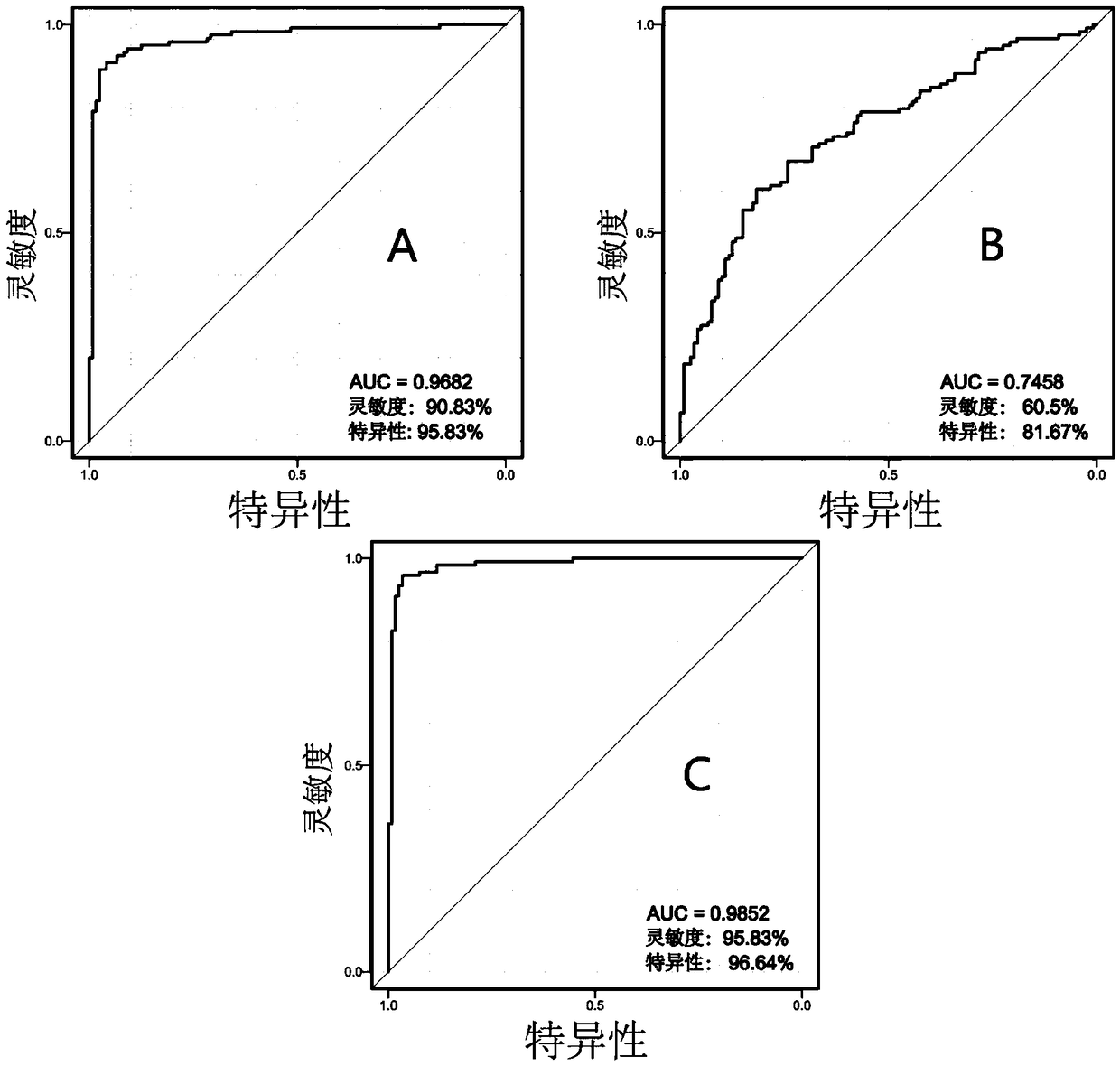
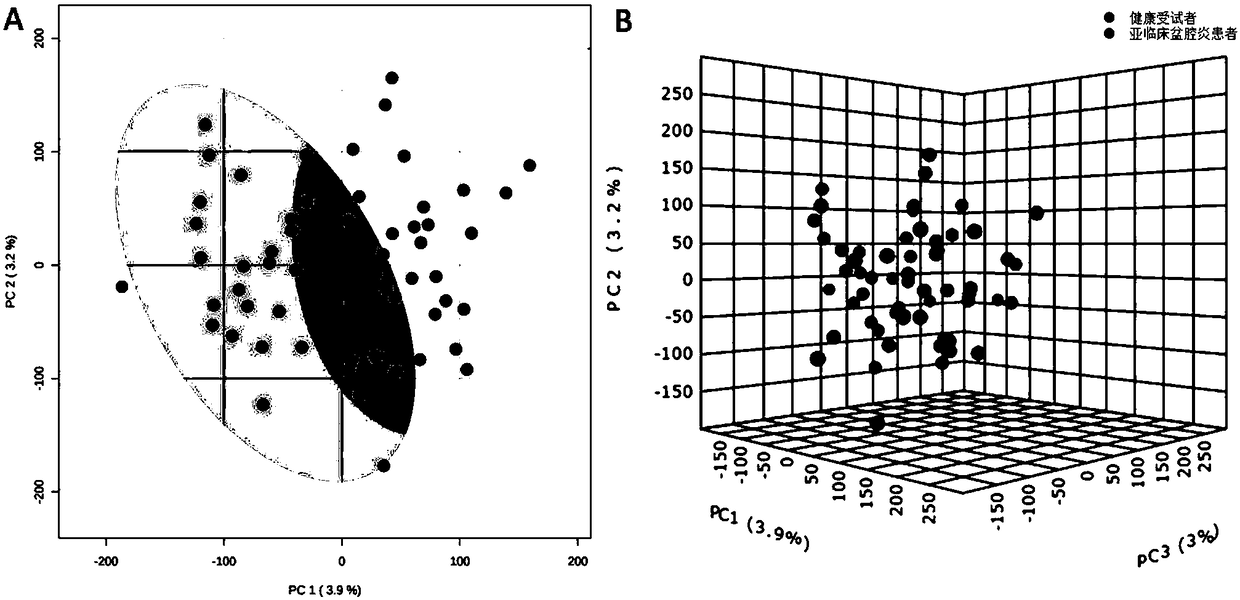
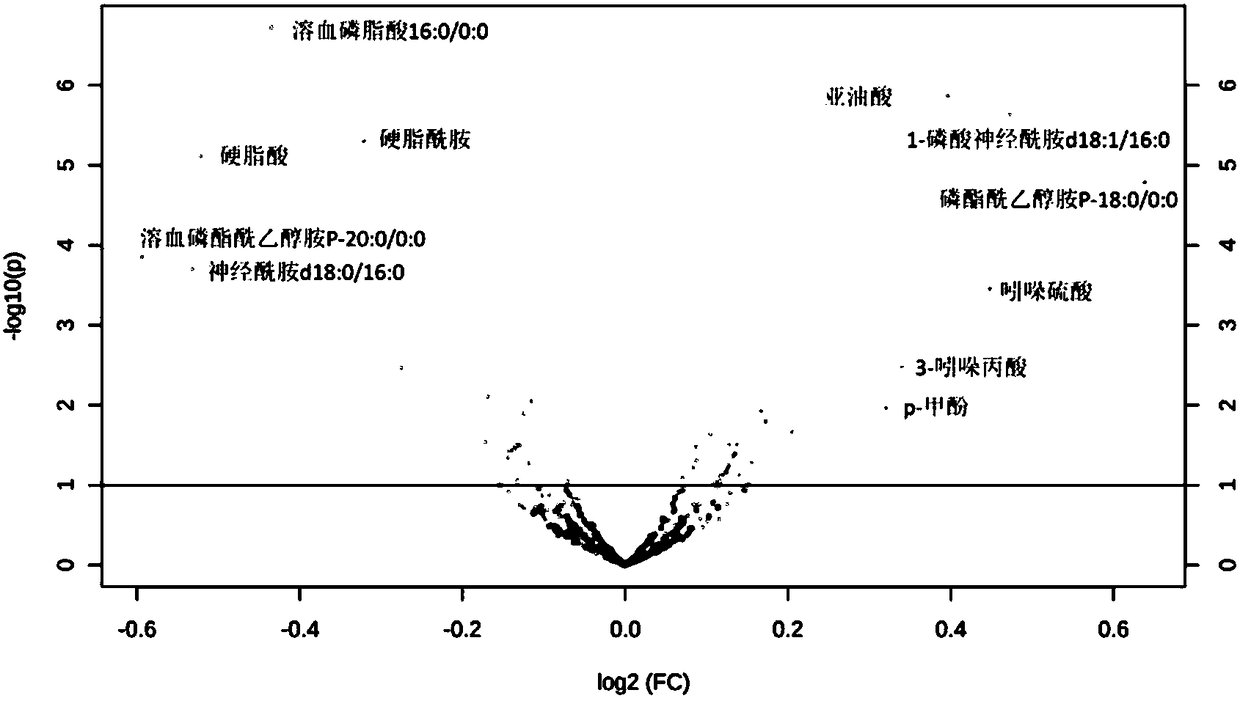
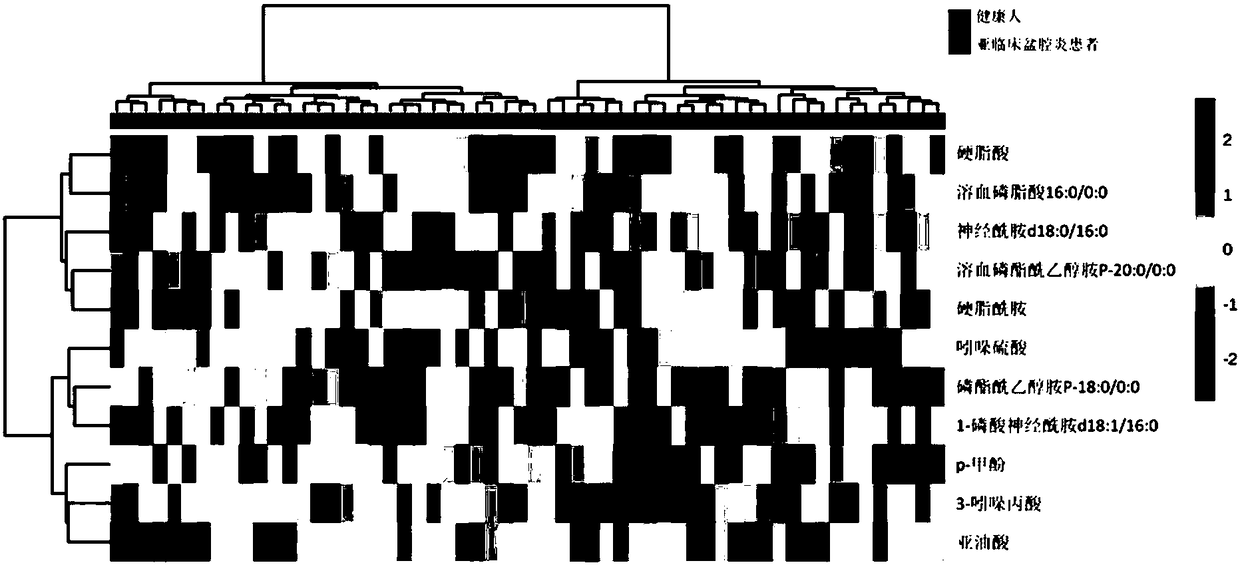
Markers for subclinical pelvic inflammation disease and applications thereof
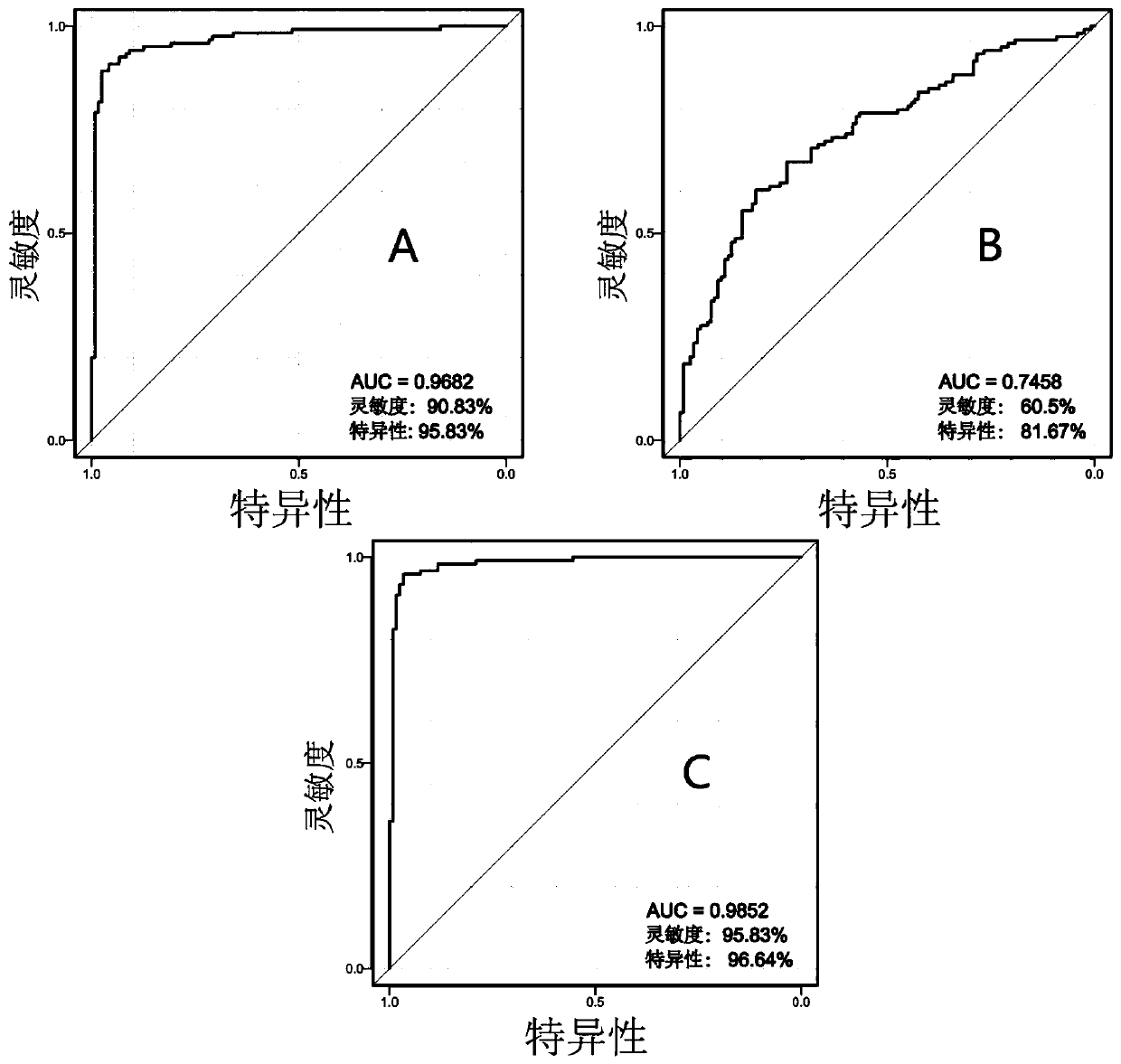
The invention belongs to the technical field of biology, and relates to a series of metabolites for diagnosis of subclinical pelvic inflammation disease. A metabonomic method based on liquid chromatogram-mass spectrometry is used for systematically screening metabolites in blood, in order to find metabolites which have obvious correlation to existence of generation of subclinical pelvic inflammation disease as biomarkers of the disease. The metabolites comprise the following components: lysobisphosphatidic acids 16:0 / 0:0, ceramide-1-phosphate d18:1 / 16:0, stearamide, linoleic acid, stearic acid, phosphatidylethanol P-18:0 / 0:0, phosphatidyl ethanolamine P-18:0 / 0:0, lysobisphosphatidyl ethanolamine P-20:0 / 0:0, indoxylsulfuric acid, 3-indolepropionic acid, and p-methylphenol. The biomarkers are used for noninvasive diagnosis and indication treatment of the subclinical pelvic inflammation disease, and compared with clinic methods for diagnosis by operation, the biomarkers have obvious advantages.
Owner:湖南省妇幼保健院
Solid preparation of doxycycline ambroxol medicine compound
InactiveCN102626392BGood effectOvercome deficienciesAntibacterial agentsPowder deliverySide effectCholesterol
The invention discloses a slow-release solid preparation of a doxycycline ambroxol medicine compound and a preparation method thereof. The preparation method comprises the following steps: preparing a vesica type phospholipid gel by adopting the following materials in parts by weight: 10 parts of doxycycline hydrochloride (count by doxycycline), 7 parts of ambroxol hydrochloride, 30-300 parts of distearoyl phosphatidylcholine ethanolamine and 10-100 parts of cholesterol succinate, and then adding frequently-used other medicinal excipients to the vesica type phospholipid gel of the doxycyclineambroxol medicine compound, thereby obtaining the slow-release solid preparation. According to the preparation method provided by the invention, the quality of preparation product is increased, the toxic side effect is reduced, the stability and dissolution rate as well as bioavailability of the medicine are greatly increased, the effect is stable and long-lasting, and the curative effect is obvious.
Owner:HAINAN YONGTIAN PHARMA INST
Stable phenyl acetic acid ester medicinal fat emulsion
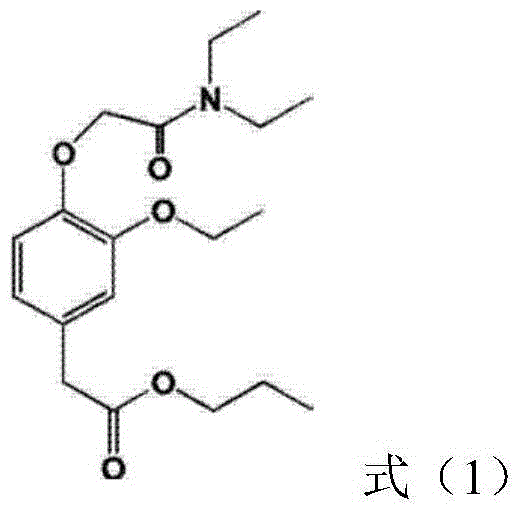
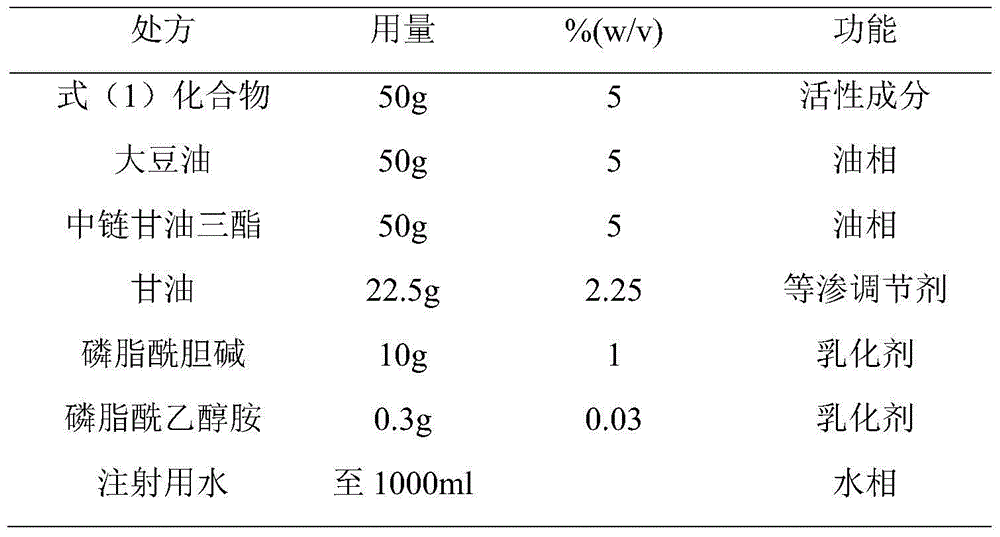
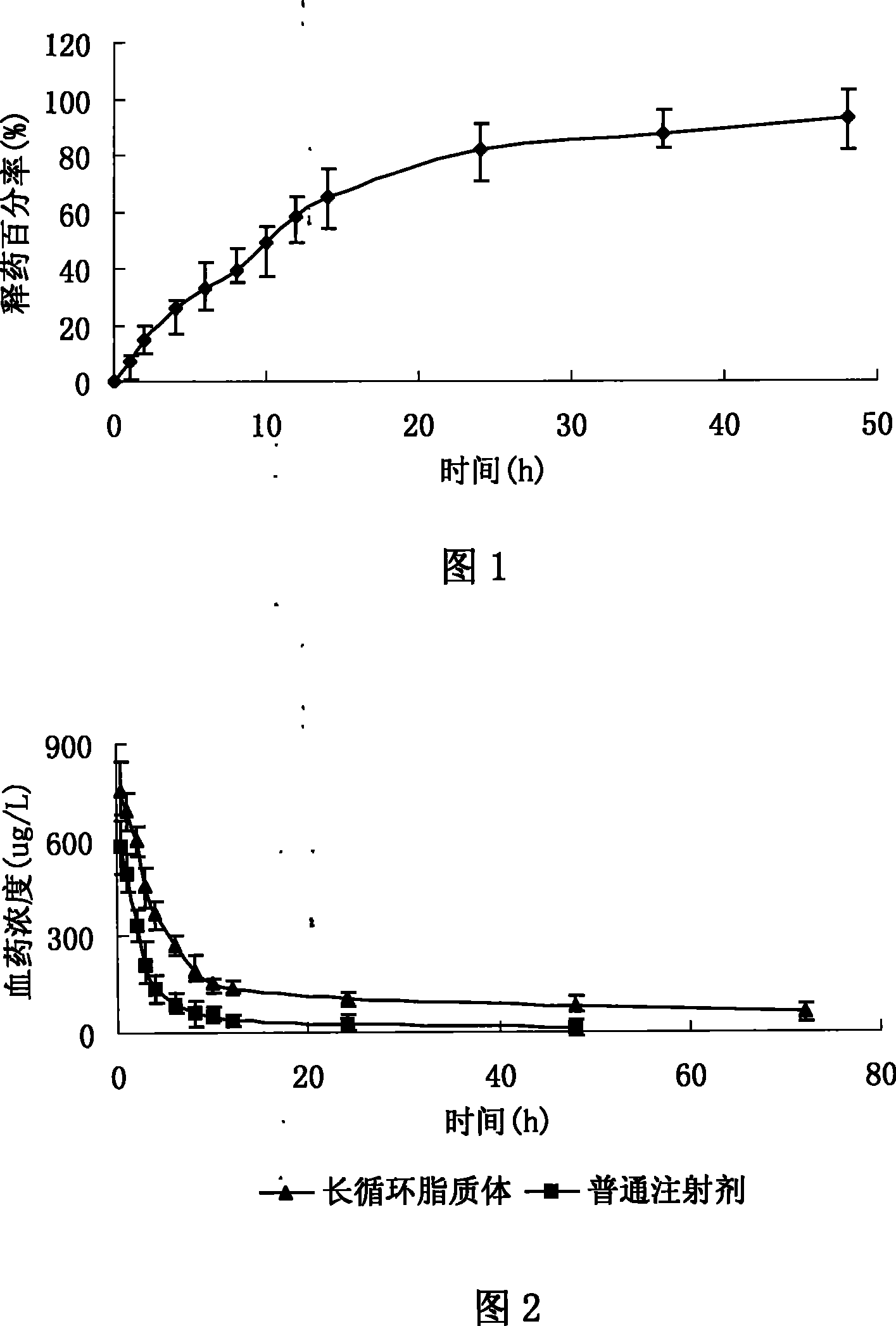
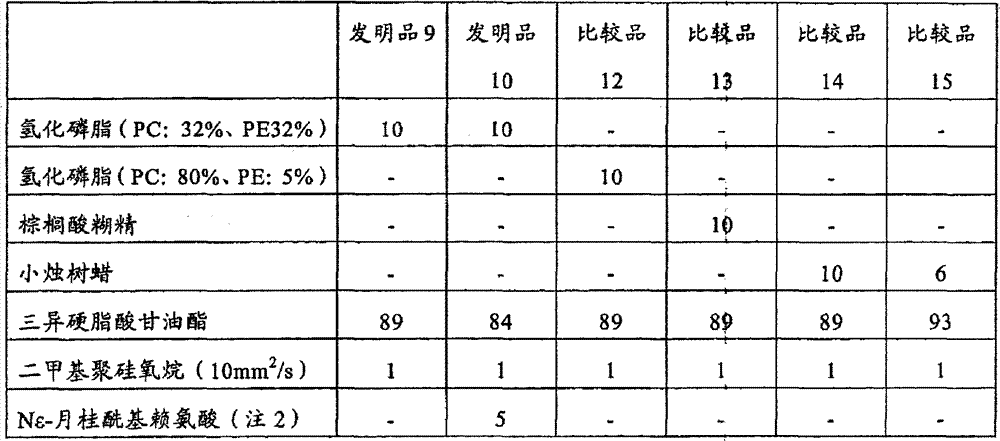
InactiveCN104887628AFix stability issuesOrganic active ingredientsAnaestheticsPhenyl acetic acidFat emulsion
The invention provides stable phenyl acetic acid ester medicinal fat emulsion which is a short-acting hypnagogue used for anesthesia and sedation. The stable phenyl acetic acid ester medicinal fat emulsion contains the following components in percentage by weight and volume: 1.0-10.0% of a phenyl acetic acid ester medicine shown in a formula (I), 10.0-30.0% of oil for injection, 0.4-1.6% of phosphatidylcholine, 0.06-0.24% of phosphatidyl ethanolamine and the balance of water for injection.
Owner:BEIJING LANDAN PHARMA TECH
Pirarubicin freeze-dry preparations and preparation method thereof
InactiveCN101181280BImprove stabilityExtend cycle timePowder deliveryOrganic active ingredientsSucroseFreeze-drying
The invention discloses a pirarubicin freeze-dried preparation. The preparation is composed of the following components with the contents: 8 to 12g of pirarubicin or pirarubicin hydrochloride, 50 to 75g of hydrogenated soybean lecithin, 28 to 42g of cholesterol, 3 to 5g of PEG2000-1, 2-dipalmitoyl-glycero-3-phosphoethanolamine, 1.2 to 2g of poloxamer 188, 32 to 50mg of Alpha-tocopherol and 450 to900g of sucrose. The preparation has good anti-cancer function, small toxicity and high stability, and the target of the drug is improved simultaneously. The invention further discloses a preparationmethod of the pirarubicin freeze-dried preparation.
Owner:深圳万乐药业有限公司
Oily solid-form cosmetic
ActiveCN102083407BEasy accessEasy to extendCosmetic preparationsHair cosmeticsPhosphatidylethanolamineSilicone oil
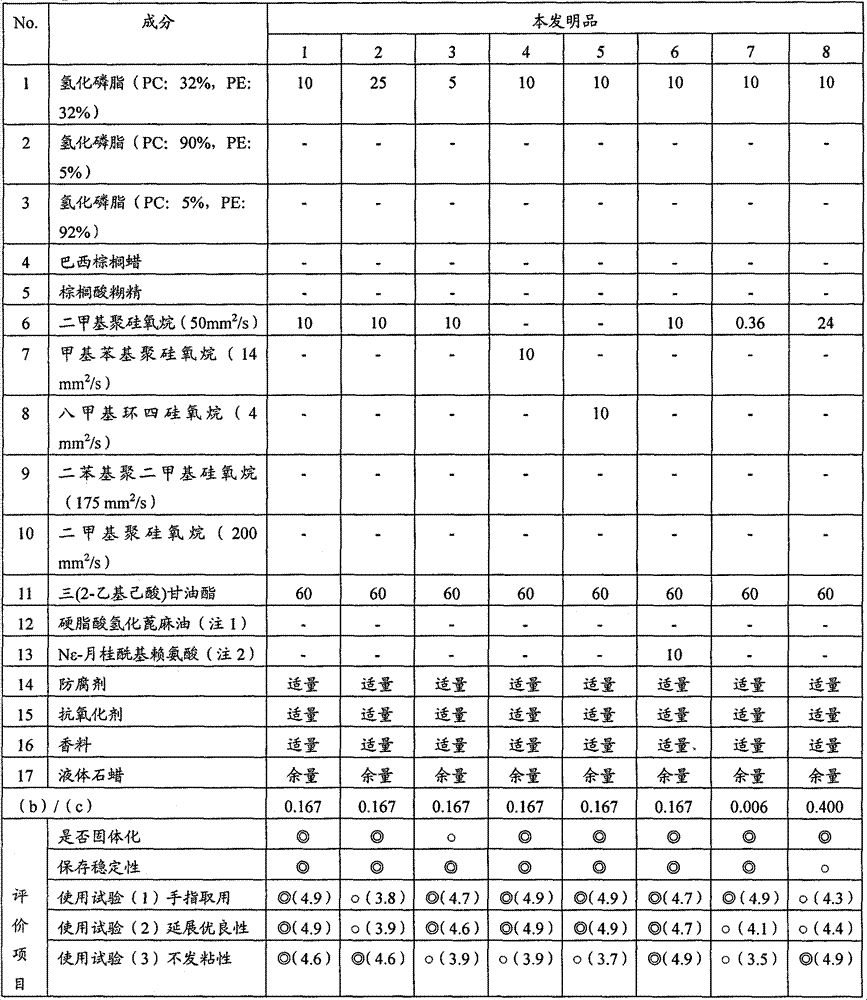
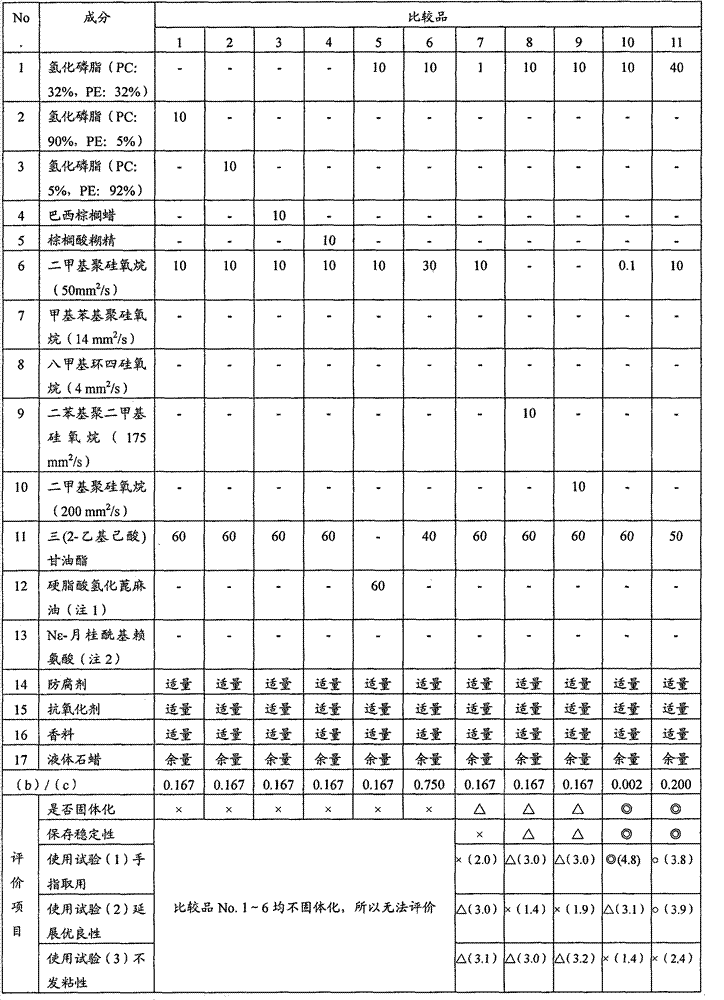
The objective is to provide an oily solid-form cosmetic, which exhibits excellent spreadability during use and when applied to the finger, reduced stickiness after application, and excellent storage stability and moisture retention. The oily solid-form cosmetic contains (a) 5-25 wt% of a phospholipid, wherein the phospholipid composition is 25-42% phosphatidylcholine and 25-42% phosphatidylethanolamine, (b) silicone oil having a kinematic viscosity of 1-100 mm2 / s at 25°C, and (c) an ester oil that is liquid at 25°C. The content ratio (b) / (c) of component (b) to component (c) is 0.006-0.4.
Owner:KOBAYASHI KOSE CO LTD
Ultrasonic contrast agent
InactiveCN105233309ALong stable timeEasy to useEchographic/ultrasound-imaging preparationsSucroseSulfur hexafluoride
The invention relates to an ultrasonic contrast agent which comprises a shell material, gas and a 6% dextran injection solution. The shell material is prepared from, by weight, 10% of dipalmitoyl phosphatidylcholine, 5% of phosphatidyl ethanolamine, 10% of polyvinylpyrrolidone, 10% of polyethylene glycol monostearate, 15% of span 80, 15% of sodium alginate, 5% of sucrose ester and 30% of injection water. The gas is prepared from perfluoro-n-butane and sulfur hexafluoride at the volume ratio of 1:3. The ultrasonic contrast agent has the advantages of being long in stable time, good in using effect, long in circulation half-life period and simple in preparing method, and has very high clinical application value.
Owner:张超
Meropenem liposome injection
InactiveCN102258487BImprove therapeutic indexImprove product qualityPowder deliveryLyophilised deliverySide effectAntioxidant
The invention discloses a meropenem liposome injection, which is characterized by mainly comprising the following components of: by weight, one part of meropenem, 2-8 parts of phosphatidylethanolamine, 1-5 parts of cholesterol, 0.2-1 part of polyether 188, 0.1-0.4 part of an antioxidant, and 5-10 parts of a supporting agent. The liposome injection prepared in the invention has good redissolving performance, good stability, high entrapment rate and little side-effect, can be used to raise the preparation product quality, minimize toxic and side effects and improve the bioavailability, and has remarkable curative effects.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
A long-circulation irinotecan liposome composition and preparation method thereof
InactiveCN103830182BHas a long cycle effectHigh tumor inhibition rateOrganic active ingredientsPowder deliverySide effectCholesterol
The invention belongs to the field of medicine preparations, which particularly relates to a long-circulation irinotecan lipidosome composition. The long-circulation irinotecan lipidosome composition is prepared from the following components in parts by weight: 1 part of irinotecan, 2 to 4 parts of hydrogenated soy phosphatidylcholine or distearoyl lecithin, 0.5 to 1.5 parts of cholesterol, 0.4 to 1.2 parts of phosphatidylethanolamine pegol and 0.0104 to 0.051 part of metal-chelator. The invention further discloses a preparation method of the long-circulation irinotecan lipidosome composition and an application of the long-circulation irinotecan lipidosome composition prepared into an irinotecan lipidosome composition injection. According to the irinotecan lipidosome composition and the preparation method thereof, the medicine loading ratio of the irinotecan is greatly improved and the encapsulation efficiency is greater than 99.5%, so that the toxic and side effect of the medicine which is not encapsulated can be obviously reduced; the content of relevant substances is lower than 0.5% and the limit of various toxic impurities is lower than 0.1%, so that the safety of the composition is obviously improved; and the composition is uniform in granularity distribution and good in stability.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Kit for detecting enzyme activity of PEMT (phosphatidyl ethanolamine N-methyltransferase)
InactiveCN105695558AEarly processing is simpleLow technical requirementsMicrobiological testing/measurementBiological material analysisS-Adenosyl-l-methionineAdenosine
The invention provides a combined product for detecting enzyme activity of PEMT (phosphatidyl ethanolamine N-methyltransferase). The combined product contains S-adenosine-L-methionine or an enzyme reaction system capable of producing S-adenosine-L-methionine, a methyl receptor and S-adenosine-L-homocysteine hydrolase, wherein the enzyme reaction system capable of producing S-adenosine-L-methionine comprises adenosine triphosphate, methionine and S-adenosylmethionine. Preferably, the combined product adopts a kit form, and all components are in self-existent reagent states. The combined product can be used on semi-automatic or full-automatic analysis detection equipment and is high in detection sensitivity, high in specificity and convenient to operate, so that the combined product can be popularized and used actually.
Owner:北京中科卓明生物医学研究所有限公司
Solid preparation of doxycycline ambroxol medicine compound
InactiveCN102626392AImprove stabilityImprove bioavailabilityAntibacterial agentsPowder deliverySide effectCholesterol
The invention discloses a slow-release solid preparation of a doxycycline ambroxol medicine compound and a preparation method thereof. The preparation method comprises the following steps: preparing a vesica type phospholipid gel by adopting the following materials in parts by weight: 10 parts of doxycycline hydrochloride (count by doxycycline), 7 parts of ambroxol hydrochloride, 30-300 parts of distearoyl phosphatidylcholine ethanolamine and 10-100 parts of cholesterol succinate, and then adding frequently-used other medicinal excipients to the vesica type phospholipid gel of the doxycyclineambroxol medicine compound, thereby obtaining the slow-release solid preparation. According to the preparation method provided by the invention, the quality of preparation product is increased, the toxic side effect is reduced, the stability and dissolution rate as well as bioavailability of the medicine are greatly increased, the effect is stable and long-lasting, and the curative effect is obvious.
Owner:HAINAN YONGTIAN PHARMA INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com