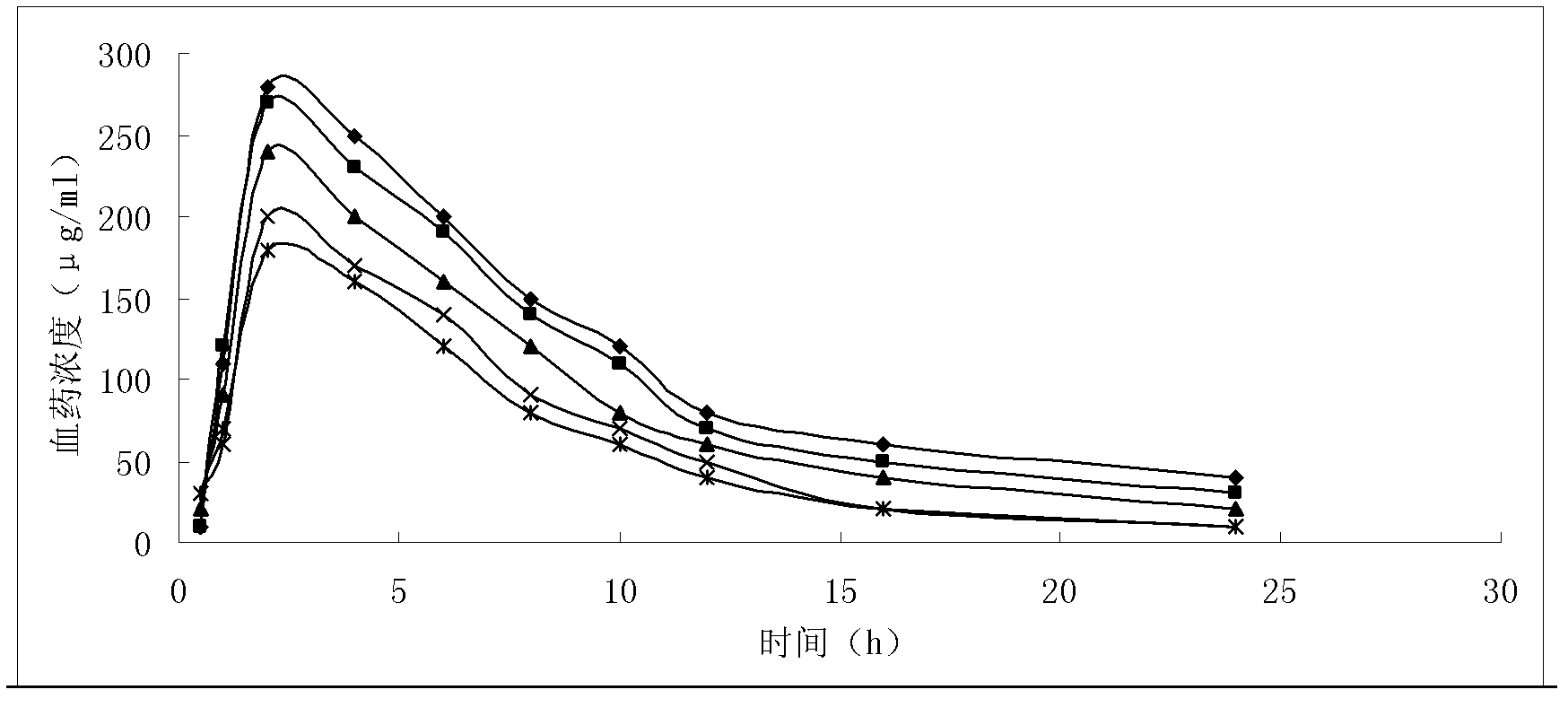
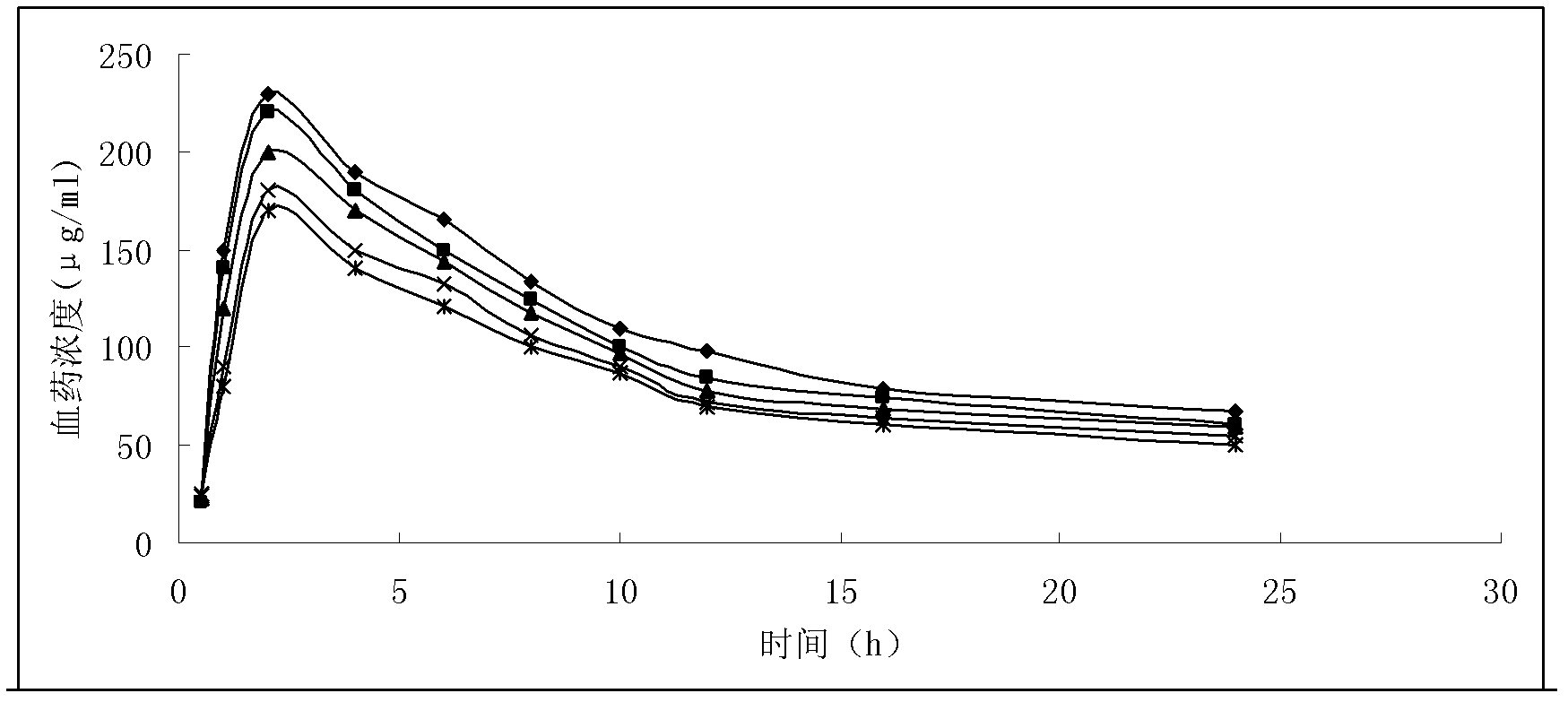
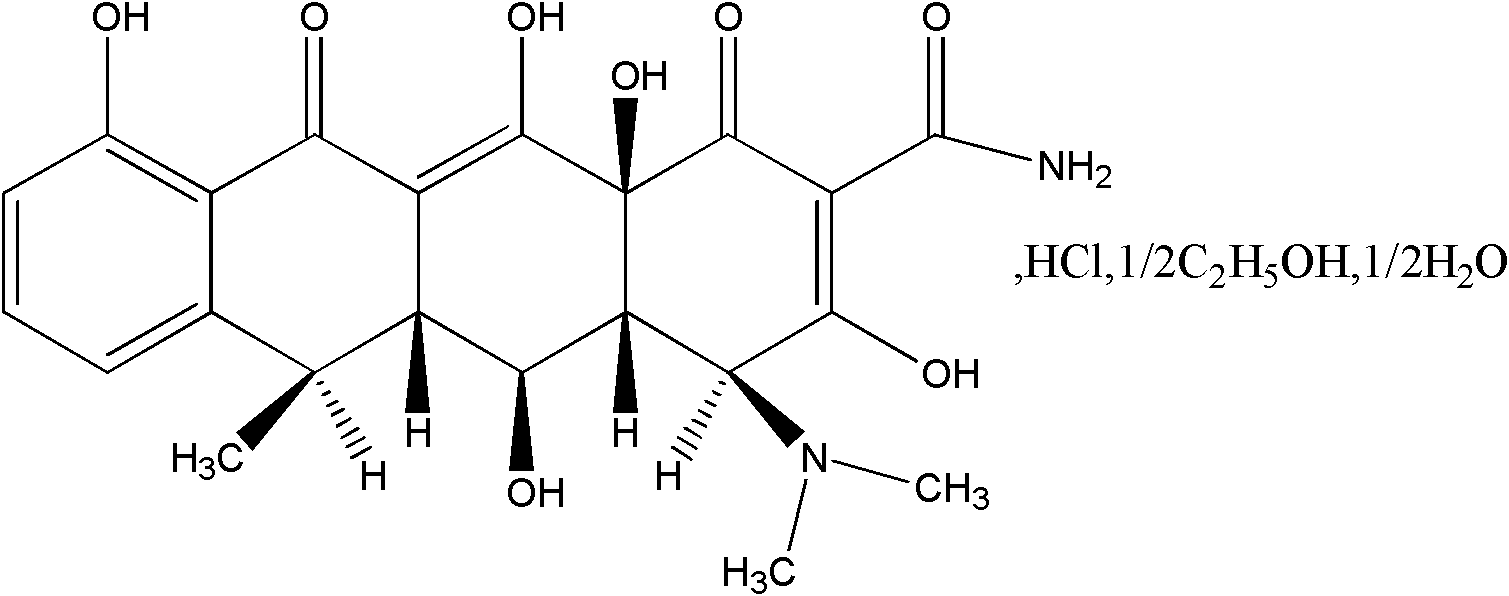
Solid preparation of doxycycline ambroxol medicine compound
A technology of doxycycline and ambroxol, applied in the field of doxycycline ambroxol pharmaceutical composition vesicular phospholipid gel solid preparation, doxycycline ambroxol composition compound preparation, can solve biological problems Problems such as low utilization, low bioavailability of sustained-release preparations, complicated preparation process, etc., to improve the dissolution of preparations, improve product quality and therapeutic effect, and reduce toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] On the other hand, the present invention provides the preparation method of doxycycline ambroxol pharmaceutical composition vesicular phospholipid gel, the method comprises the following steps:
[0068] (1) Dissolve distearoylphosphatidylethanolamine, cholesterol succinate and ambroxol hydrochloride in an appropriate amount of organic solvent under nitrogen protection, and stir magnetically for 1-2 hours at about 50° C. to obtain a phospholipid solution;
[0069] (2) Filter the above-mentioned phospholipid solution with a 0.45 μm microporous membrane, freeze the filtrate at -50°C for 4 hours, then raise the temperature to 10°C at a rate of 3°C / hour for 2 hours, and then repeatedly freeze Melt three times, and finally dry at 25°C for 1-3 hours until it is completely dry to obtain a dry solid in the form of a loose sponge;
[0070] (3) Disperse the above solid and doxycycline hydrochloride in a phosphate buffer solution of pH 6.8 under the protection of nitrogen, stir it ...
Embodiment 1
[0109] The preparation of embodiment 1 doxycycline ambroxol pharmaceutical composition vesicular phospholipid gel tablet
[0110] The raw and auxiliary materials used in the prescription (1000 tablets) are as follows:
[0111]
[0112] Preparation Process
[0113] (1) under nitrogen protection, 120g distearoyl phosphatidylethanolamine and 40g cholesterol succinate and 7g ambroxol hydrochloride are dissolved in the mixed solvent of n-butanol and tert-butyl alcohol that the volume ratio of 250ml is 2: 3, Stir magnetically for 2 hours at about 50°C to obtain a phospholipid solution;
[0114] (2) Filter the above-mentioned phospholipid solution with a 0.45 μm microporous membrane, freeze the filtrate at -50°C for 4 hours, then raise the temperature to 10°C at a rate of 3°C / hour for 2 hours, and then repeatedly freeze Melt three times, and finally dry at 25°C for 1 hour until it is completely dry to obtain a dry solid in the form of a loose sponge;
[0115] (3) Disperse the a...
Embodiment 2
[0119] The preparation of embodiment 2 doxycycline ambroxol pharmaceutical composition vesicular phospholipid gel tablet
[0120] The raw and auxiliary materials used in the prescription (1000 tablets) are as follows:
[0121]
[0122]
[0123] Preparation Process
[0124] (1) under nitrogen protection, 180g distearoylphosphatidylethanolamine and 60g cholesteryl succinate and 7g ambroxol hydrochloride are dissolved in the mixed solvent of n-butanol and tert-butyl alcohol that the volume ratio of 200ml is 2:3, Stir with magnetic force for 1 hour at about 50°C to obtain a phospholipid solution;
[0125] (2) Filter the above-mentioned phospholipid solution with a 0.45 μm microporous membrane, freeze the filtrate at -50°C for 4 hours, then raise the temperature to 10°C at a rate of 3°C / hour for 2 hours, and then repeatedly freeze Melt three times, and finally dry at 25°C for 3 hours until completely dry to obtain a dry solid in the form of a loose sponge;
[0126] (3) Dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com