Officinal magnolia phenol lipid frozen dried powder preparation and its use in preparing drug for cancers
A technology of freeze-dried powder and preparation, applied in the directions of anti-tumor drugs, freeze-dried delivery, drug combination, etc., can solve the problems of not improving pharmacokinetics, unable to prolong the circulation time of honokiol, etc. Anti-tumor function, good sensitization and synergistic effect, effect of improving absorption and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
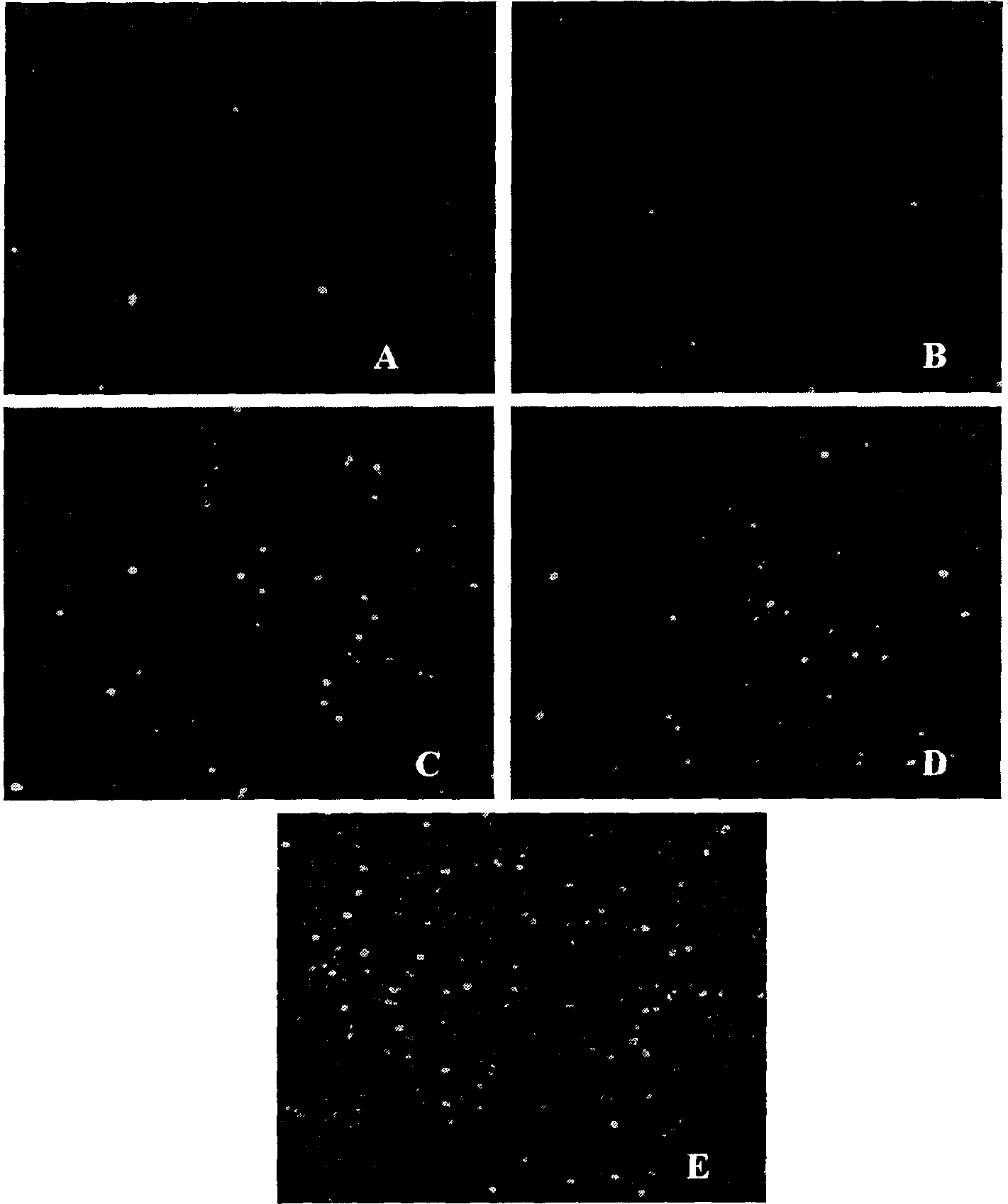
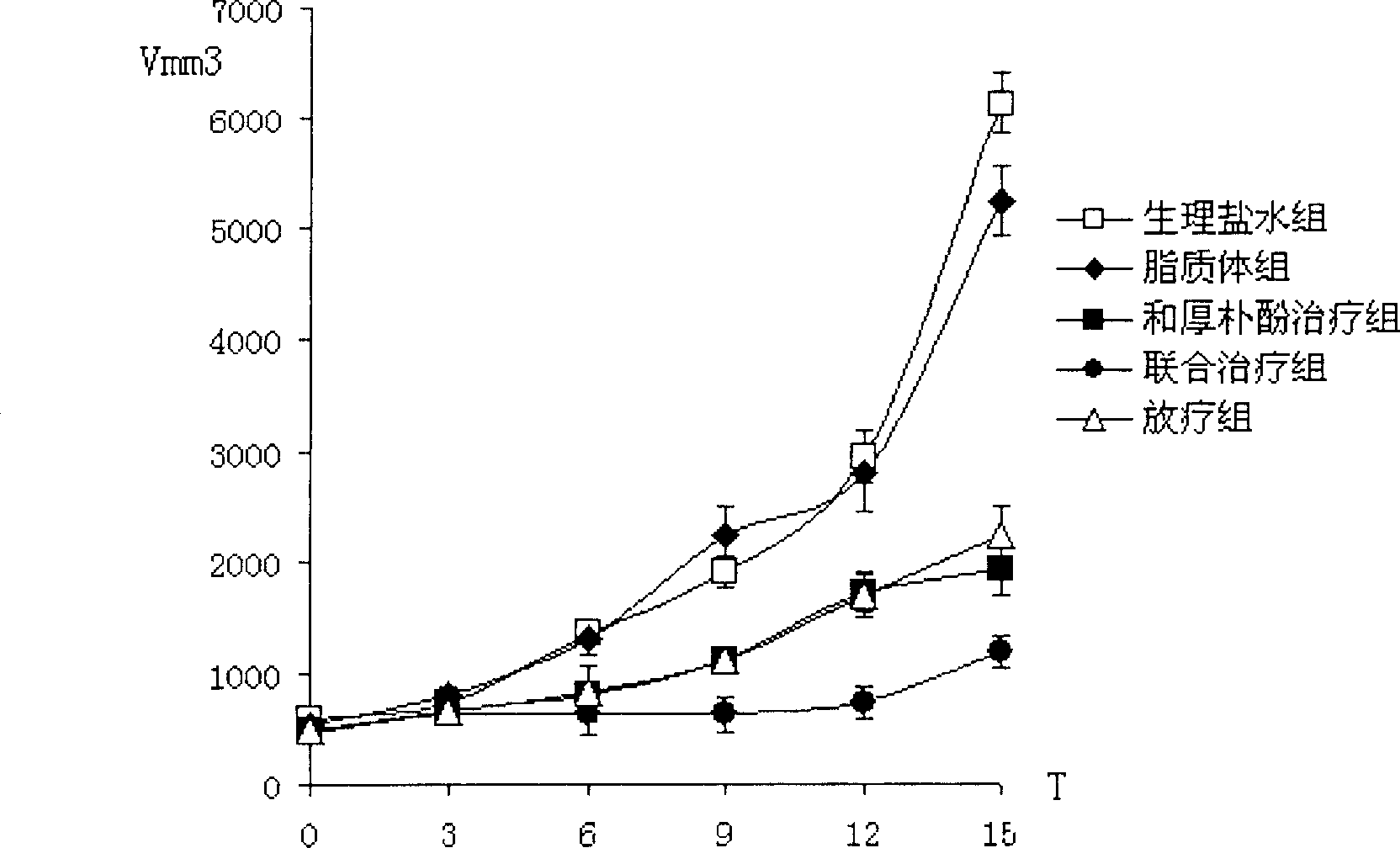
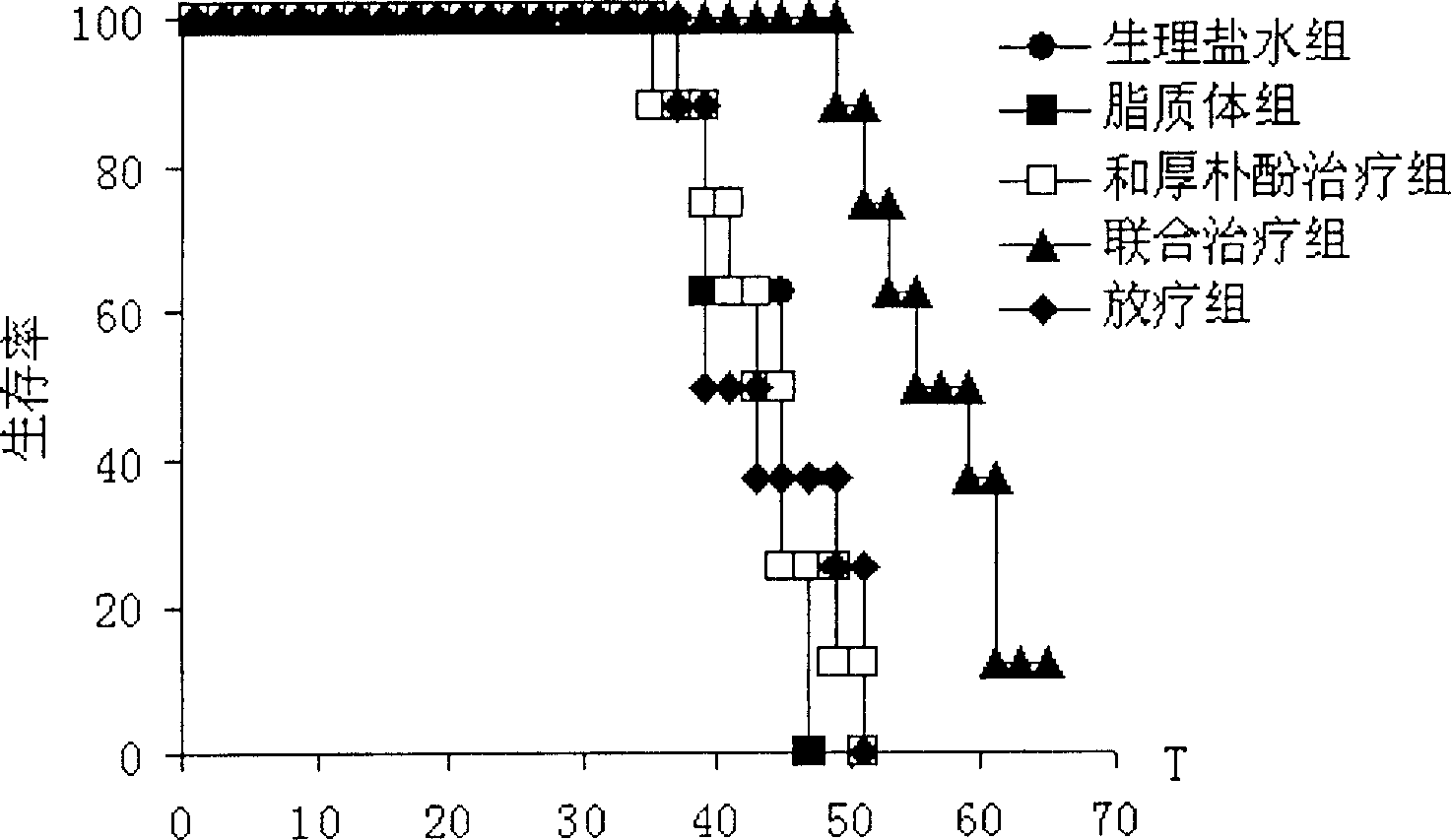
Image
Examples
Embodiment 1
[0081] Weigh 20 mg of honokiol, 60 mg of lecithin, 8 mg of cholesterol, 12 mg of polyethylene glycol-phosphatidylethanolamine with a molecular mass of polyethylene glycol of 2000, 1 mg of sorbitol, and 0.1 mg of vitamin C;
[0082] The above-mentioned weighed lecithin, cholesterol, polyethylene glycol-phosphatidylethanolamine and honokiol are dissolved in an organic solvent of 100ml of chloroform and methyl alcohol prepared in proportion, wherein chloroform is 75ml , methanol is 25ml, stir in a closed bottle until it is completely dissolved, put the completely dissolved solution in a 250ml flask, connect the flask to a rotary evaporator, immerse the flask in a constant temperature water bath at 40°C, and vacuum the rotary evaporation to remove organic matter. Solvent, at this moment, form a uniform layer of phospholipid film on the wall of the flask, then put it into a vacuum drying oven, dry it at room temperature for 2 hours, add 100ml of sterilized water, and carry out ultra...
Embodiment 2
[0084] Weighing honokiol 35mg, lecithin 45mg, cholesterol 12mg, polyethylene glycol molecular weight 4000 polyethylene glycol-phosphatidylethanolamine 8mg, sorbitol 0.5mg, vitamin C 0.5mg;
[0085] Honokiol liposome freeze-dried powder was prepared according to the conditions of use and operation steps in Example 1, which was vacuumized or filled with an inert gas to seal, and stored at a temperature of 4°C-8°C for future use.
Embodiment 3
[0087] Weigh honokiol 50mg, lecithin 40mg, cholesterol 5mg, polyethylene glycol molecular weight 8000 polyethylene glycol-phosphatidylethanolamine 5mg, glucose 4mg, vitamin C 0.1mg;
[0088] Honokiol liposome freeze-dried powder was prepared according to the conditions of use and operation steps in Example 1, which was vacuumized or filled with an inert gas to seal, and stored at a temperature of 4°C-8°C for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com