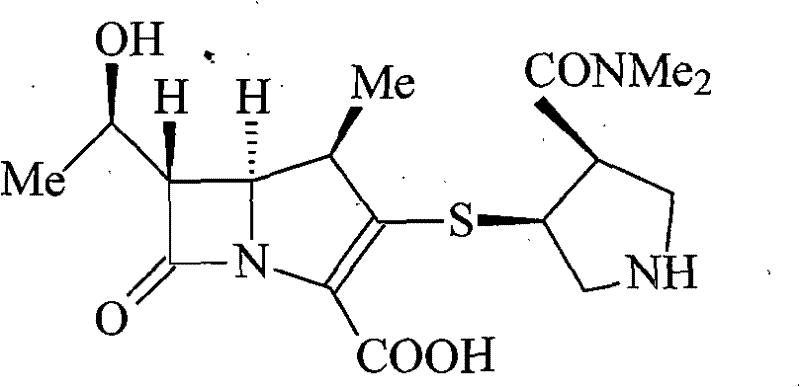
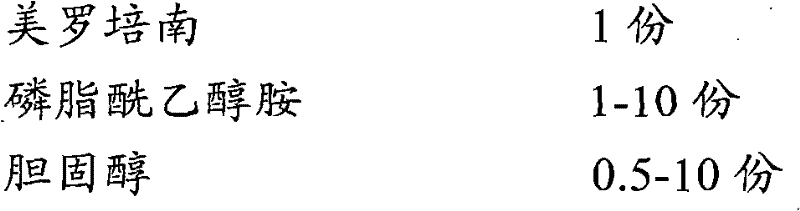A kind of meropenem liposome injection
A technology of meropenem and liposome, which is applied in the directions of non-active ingredients medical preparations, organic active ingredients, powder delivery, etc., can solve the problems of low bioavailability of meropenem, and improve the quality of preparation products and the encapsulation efficiency. The effect of high and improved therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Meropenem liposome injection
[0036] Prescription (1000 bottles):
[0037]
[0038] Production Process:
[0039] (1) Add 25g of meropenem, 100g of phosphatidylethanolamine, 125g of cholesterol, 5g of tert-butyl-p-hydroxyanisole and 25g of polyether 188 into 800ml of a mixed solvent of ethanol and acetone with a volume ratio of 3:1, heat and stir to disperse evenly , remove ethanol and acetone under reduced pressure on a rotary evaporator to prepare a phospholipid film;
[0040] (2) Add 800ml of carbonate buffer solution with a pH of 5.6, shake and stir to completely hydrate the phospholipid membrane, high-speed homogeneously emulsify, and filter through a microporous membrane to prepare a meropenem liposome suspension;
[0041] (3) Add 250 g of PVP to the above liposome suspension, keep warm at 40-50°C and stir for 20-30 minutes to dissolve, then cool to room temperature to obtain a meropenem liposome solution;
[0042] (4) The above solution was freeze...
Embodiment 2
[0043] Example 2 Preparation of meropenem liposome injection
[0044] Prescription (1000 bottles):
[0045]
[0046] Preparation Process:
[0047] (1) Add 50g of meropenem, 300g of phosphatidylethanolamine, 150g of cholesterol, 10g of tert-butyl-p-hydroxyanisole and 40g of polyether 188 into 1000ml of a mixed solvent of ethanol and acetone with a volume ratio of 3:1, heat and stir to disperse evenly , remove ethanol and acetone under reduced pressure on a rotary evaporator to prepare a phospholipid film;
[0048] (2) Add 1000ml of carbonate buffer solution with a pH of 5.6, shake and stir to completely hydrate the phospholipid membrane, high-speed homogeneously emulsify, and filter through a microporous membrane to prepare a meropenem liposome suspension;
[0049] (3) Add 300 g of PVP to the above liposome suspension, keep warm at 40-50°C and stir for 20-30 minutes to dissolve it, then cool to room temperature to obtain a meropenem liposome solution;
[0050] (4) The above...
Embodiment 3
[0051] Example 3 Preparation of meropenem liposome injection
[0052] Prescription (1000 bottles):
[0053]
[0054] Preparation Process:
[0055] (1) Add 100g of meropenem, 200g of phosphatidylethanolamine, 100g of cholesterol, 10g of tert-butyl-p-hydroxyanisole and 20g of polyether 188 into 800ml of a mixed solvent of ethanol and acetone with a volume ratio of 3:1, heat and stir to disperse evenly , remove ethanol and acetone under reduced pressure on a rotary evaporator to prepare a phospholipid film;
[0056] (2) Add 800ml of carbonate buffer solution with a pH of 5.6, shake and stir to completely hydrate the phospholipid membrane, high-speed homogeneously emulsify, and filter through a microporous membrane to prepare a meropenem liposome suspension;
[0057] (3) Add 500 g of PVP to the above liposome suspension, keep warm at 40-50°C and stir for 20-30 minutes to dissolve, then cool to room temperature to obtain a meropenem liposome solution;
[0058] (4) The above s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com