Efficient and economic asymmetric synthesis of nootkatone, tetrahydronootkatone, their precursors and derivatives
A technology of tetrahydronootkatone and dihydronootkatone, which is applied in the preparation of organic compounds, organic chemistry, organic chemical methods, etc., can solve the problems of lengthy synthesis steps, hindering commercial application, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
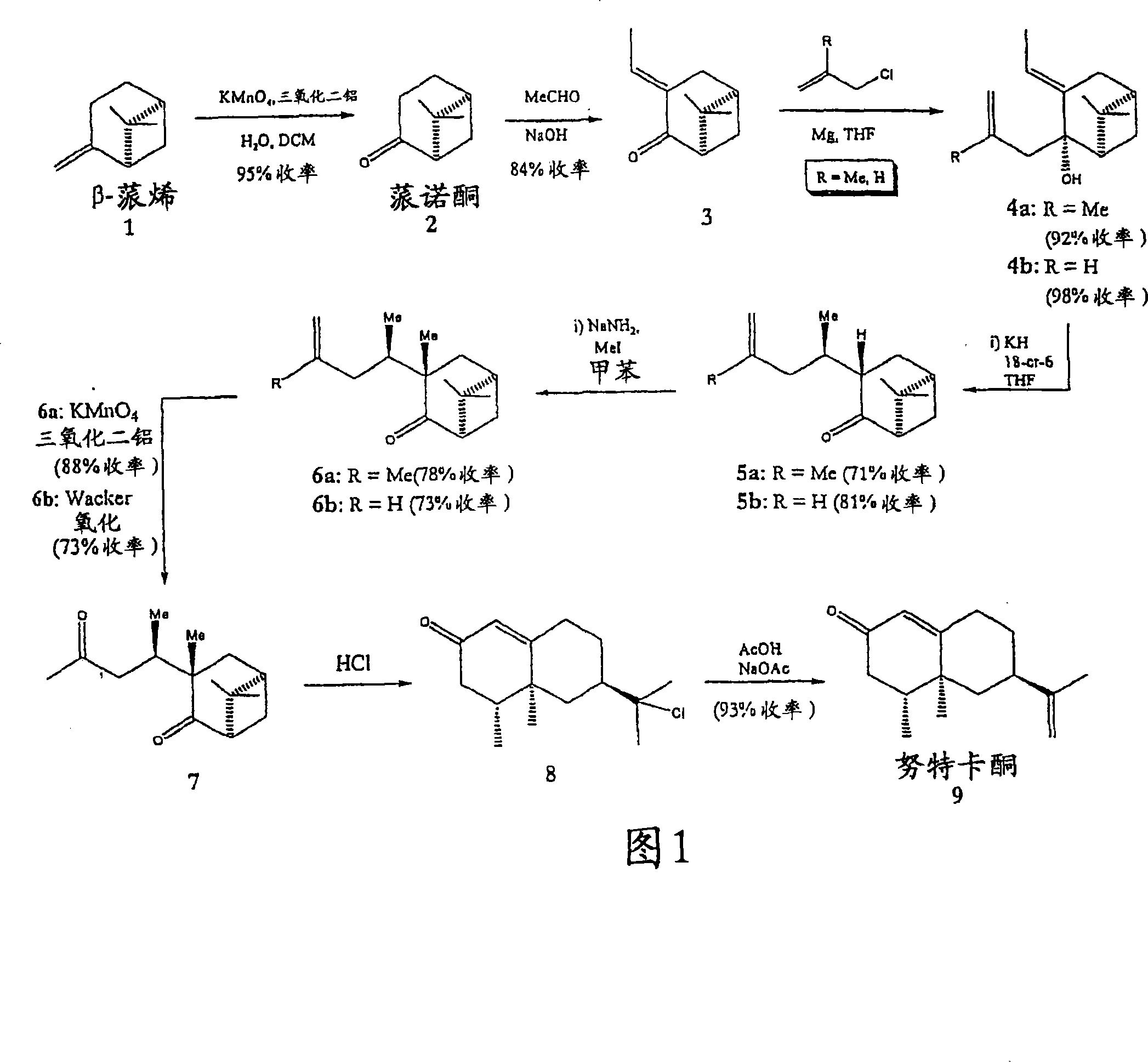
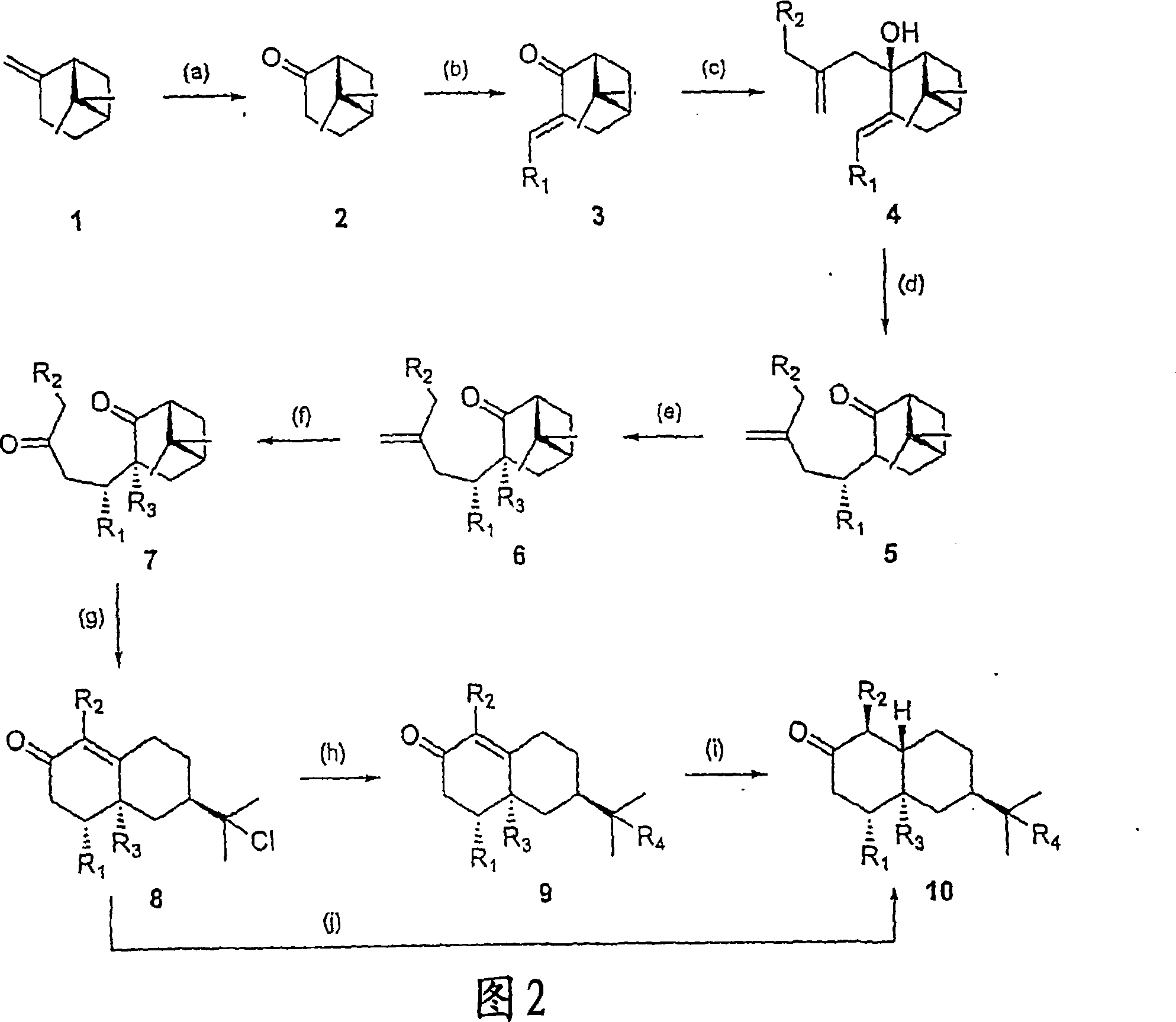
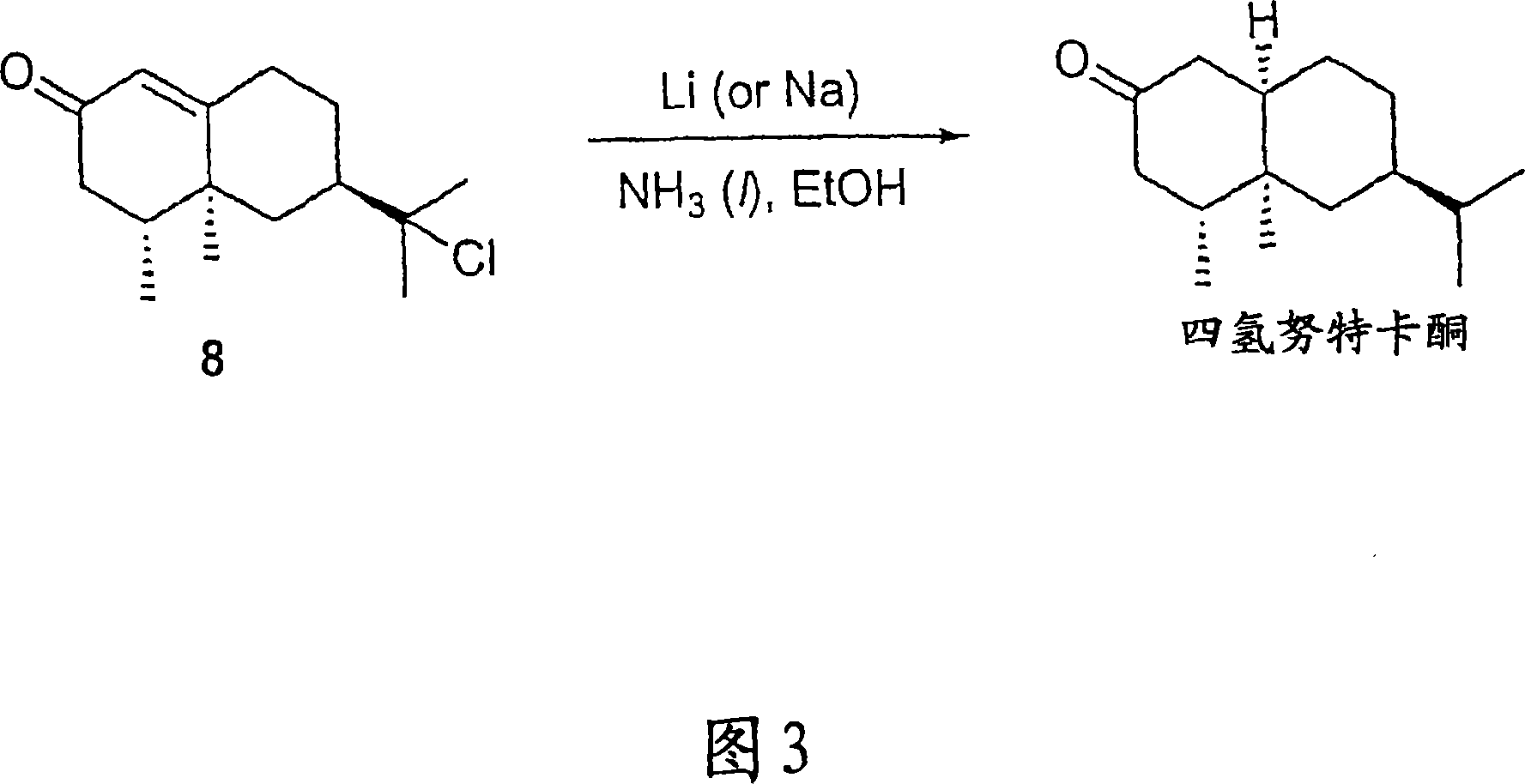
[0028]6,6-Dimethyl-bicyclo[3.3.1]heptan-2-one, nopinone (compound 2): finely ground KMnO4 (2.8 g, 17.8 mmol), acidic alumina (Brockmann Activity 1, 11.2 g, 0.1098 mol) and water (2.79 g, 0.1552 mol) were mixed for 5 minutes to give a homogeneous mixture. Commercially available (-)-β-pinene (0.5 g, 0.582 ml, 3.67 mmol) was dissolved in dichloromethane (DCM) (100 mL), and the solution was placed in a round bottom flask. To the above solution was added the permanganate / alumina mixture in small portions over 10 minutes while continuing to stir. The reaction was allowed to proceed at room temperature and the progress of the reaction was monitored by TLC (90:10 / Hexane:EtOAc). After reaction of essentially all starting material was complete, the crude mixture was filtered through a fritted glass funnel and the residue was washed with DCM (2 x 50 mL). Excess solvent was removed by rotary evaporator to give a yellow oil, which was further purified by column chromatography (90:10 / hexa...
Embodiment 2
[0030] (1R,5R)-6,6-Dimethyl-3-(E)-ethylidenebicyclo[3.3.1]heptan-2-one (Compound 3): Place a magnetic stirring bar on a device equipped with a constant pressure Addition funnel and 2 inlet valves in a clean, dry three necked jacketed round bottom flask. The flask was then purged with argon. Compound 2 (1 g, 1.0194 ml, 7.24 mmol) and KOH (0.4872 g, 8.7 mmol) were dissolved in ethanol (17.2 mL) in a flask under argon. The resulting solution was cooled to 5°C. Still under argon, a solution of acetaldehyde (0.609 mL, 0.4781 g, 10.9 mmol) in EtOH (4.3 mL) was added to the flask over 30 minutes. The mixture was reacted at 5°C for 15 hours. An additional 4 parts of acetaldehyde (0.609 mL) in EtOH (4.3 mL) was added to the reaction mixture at 15 hour intervals, maintained at 5 °C. Stirring was continued for 6 hours after addition of the last portion of acetaldehyde and EtOH. A solution of p-toluenesulfonic anhydride (1.927 g, 10.1 mmol) in EtOH (5 mL) was then added to the mixtur...
Embodiment 3
[0033] 3-Ethylidene-6,6-dimethyl-2-(2-methyl-allyl)-bicyclo[3.1.1]heptan-2-ol (compound 4a): at 60°C, 30 A solution of methallyl chloride (0.692 g, 7.64 mmol) in freshly distilled tetrahydrofuran (THF) (2.5 mL) was added to flame-dried Mg metal (0.28 g, 11.5 mmol) within minutes. THF (2.5mL) suspension. The resulting Grignard solution darkened during an additional 20 minutes at reflux. The mixture was then cooled to -42°C (dry ice / chlorobenzene bath), and a solution of enone compound 3 (0.4182 g, 2.6 mmol) in THF (2.5 mL) was added dropwise. After 5 minutes the cooling bath was removed and the reaction was stirred for 1.5 hours while allowing to warm to room temperature. The mixture was then poured into ice-cold 0.1N HCl (50 mL) and extracted with ether. The combined organic fractions were washed with water and brine, Na 2 SO 4 Dry, filter and concentrate. Column chromatography (90:10 / Hexane:EtOAc) afforded compound 4a (0.52 g, 92% yield) as a colorless liquid. 1 HNMR: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com