Gluconic acid modified chitosan nucleophilic NO donator and synthesizing method thereof
A technology of gluconic acid and chitosan, which is applied in the field of medical engineering, can solve the problems of small load and poor targeting, and achieve the effect of large load, prevention of restenosis, and promotion of wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
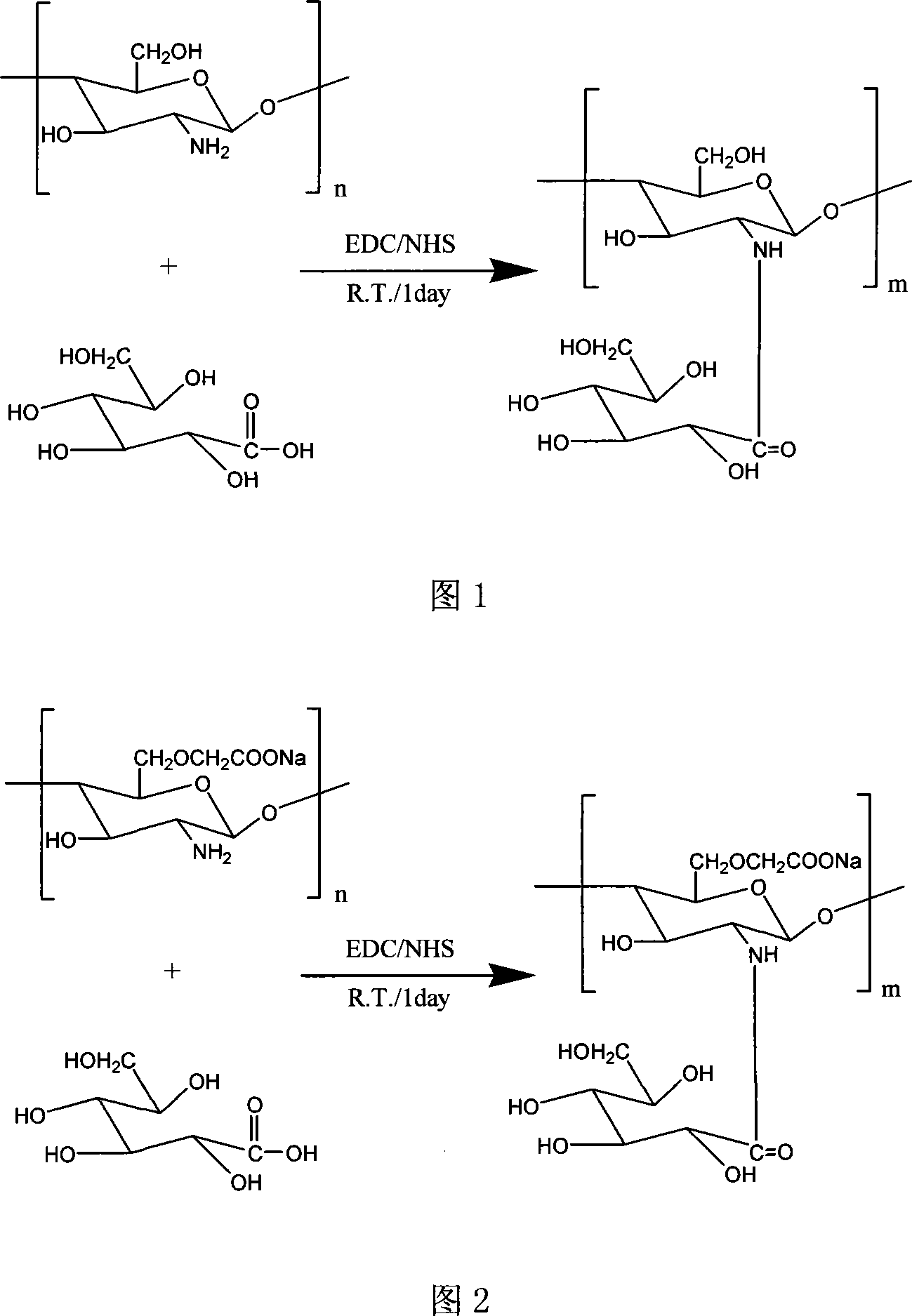
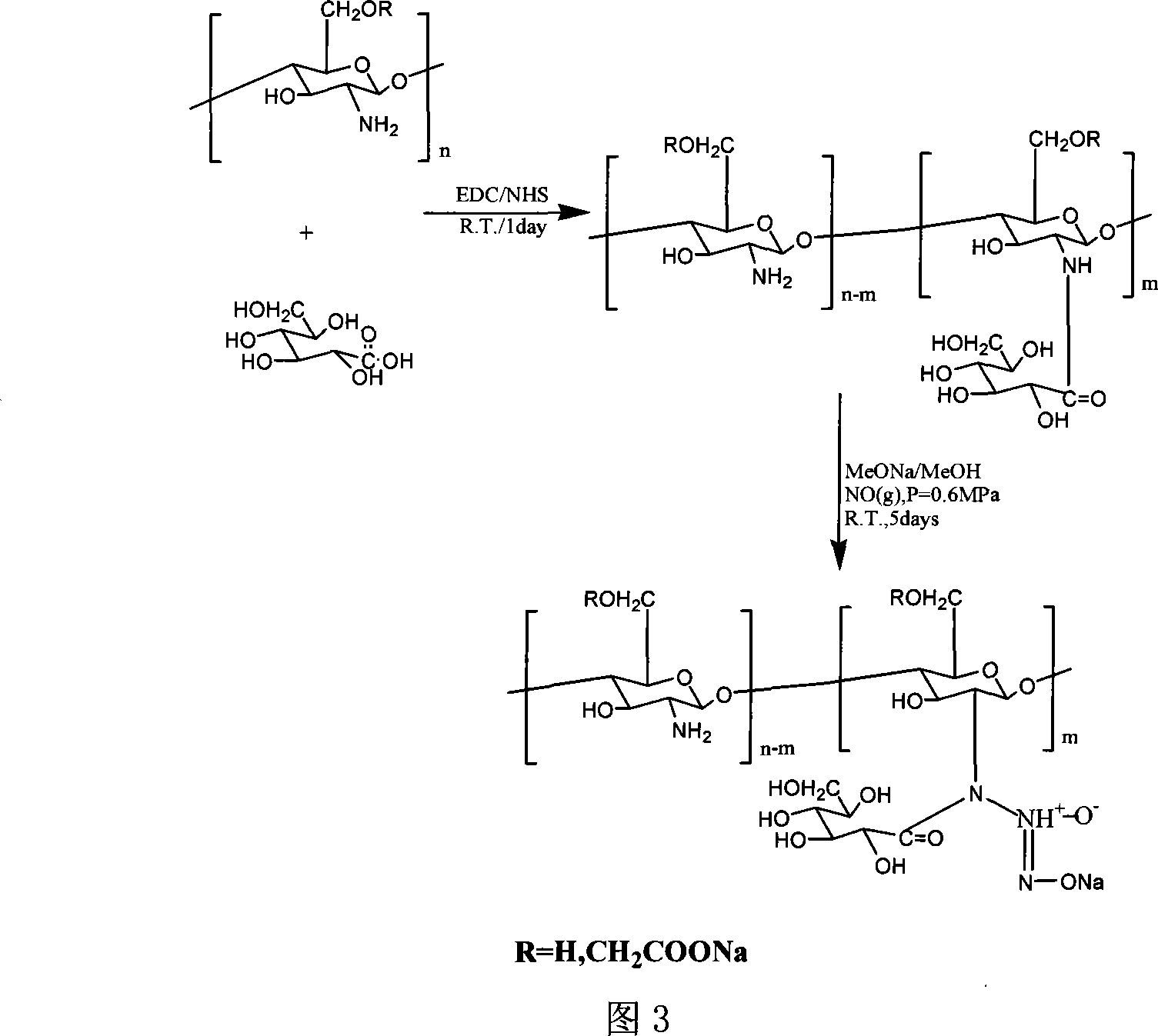
[0026] Embodiment 1: the synthesis of gluconic acid modified 400,000 molecular weight chitosan / NO
[0027]400,000 molecular weight chitosan (1 g) and gluconic acid (0.1 or 0.2 equal parts / [-NH2]) were dissolved in 0.1N HCl hydrochloric acid solution (100 ml), then EDC (1.5 equal parts / gluconic acid) was added to the solution and NHS (0.25 equal parts / EDC), then adjust the pH of the solution to 5 with a solution of 1N NaOH / 1N HCl, and continue to stir the reaction solution at room temperature for 24 hours. After the reaction, adjust the pH to 9 with 1N NaOH solution, and the reaction solution The product was dialyzed with distilled water for 5 days in a dialysis bag and dried to obtain a colorless transparent film-like substance SBC (1.44 g).
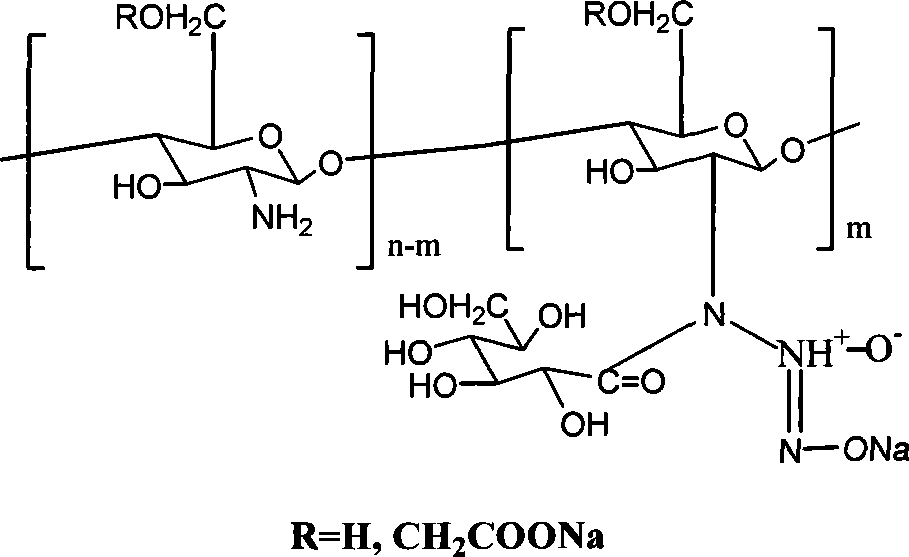
[0028] Add 0.23g (0.001mol) of the above reaction product into 100ml of anhydrous methanol solution containing 0.22g (0.002mol) sodium methoxide, react with NO in an autoclave, maintain the pressure at 5atm, and react for 3 days. Wash w...
Embodiment 2
[0030] Embodiment 2: the synthesis of gluconic acid modified 1.24 million molecular weight 0-carboxymethyl chitosan / NO
[0031] O-carboxymethyl chitosan (1 g) with a molecular weight of 1.24 million and gluconic acid (0.1 or 0.2 equal parts / [-NH2]) were dissolved in 0.1N HCl hydrochloric acid solution (100 ml), and EDC (1.5 Equal parts / gluconic acid) and NHS (0.25 equal parts / EDC), then use 1N NaOH / 1N HCl solution to adjust the pH of the solution to 5, and the reaction solution continues to stir and react at room temperature for 24h. After the reaction is completed, use 1N NaOH solution to prepare pH=9, the reaction solution was dialyzed with distilled water for 5 days in a dialysis bag, and the product was obtained after drying, which was N-gluconic acid-O-carboxymethyl chitosan SBCS (1.33g) in the form of pink fine powder.
[0032] Add 0.32g (0.001mol) of the above reaction product SBCS into 100ml of anhydrous methanol solution containing 0.22g (0.002mol) sodium methoxide, r...
Embodiment 3
[0034] The synthesis of embodiment 3 gluconic acid modified 1.88 million molecular weight chitosan / NO
[0035] Chitosan (1 g) with a molecular weight of 1.88 million and gluconic acid (0.1 or 0.2 equal parts / [-NH2]) were dissolved in 0.1N HCl hydrochloric acid solution (100 ml), then EDC (1.5 equal parts / gluconic acid ) and NHS (0.25 equal parts / EDC), then use 1N NaOH / 1N HCl solution to adjust the pH of the solution to 5, and the reaction solution is stirred at room temperature for 24 hours. After the reaction is completed, adjust the pH to 9 with 1N NaOH solution. The liquid was dialyzed with distilled water for 5 days in a dialysis bag, and the product was obtained after drying, and a white opaque film-like substance SBC (1.20 g) was obtained.
[0036] Add 0.20 g (0.001 mol) of the above reaction product into 100 ml of anhydrous methanol solution containing 0.22 g (0.002 mol) of sodium methylate, react with NO in an autoclave, maintain a pressure of 10 atm, and react for 7 d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com