Salt of imidazole-carboxylic acid derivant, production method and pharmaceutical composition thereof
A pharmacy, general formula technology, applied in the direction of drug combination, active ingredients of heterocyclic compounds, and medical preparations containing active ingredients, etc., can solve the problems of poor compound solubility, complicated preparation process and insoluble
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
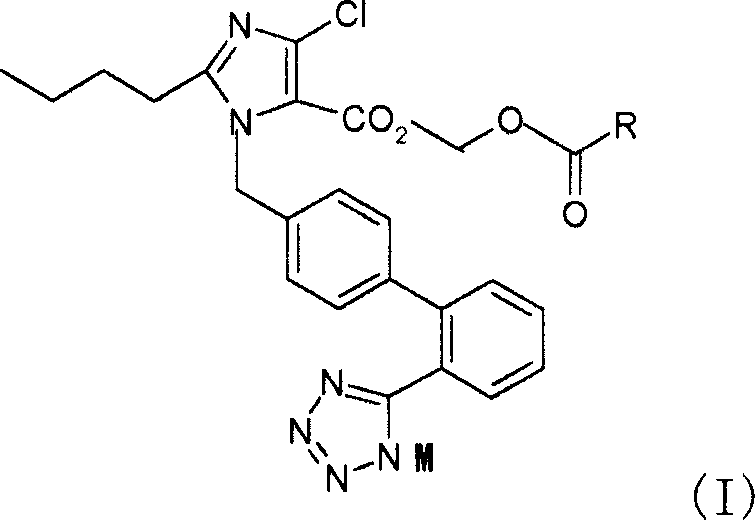
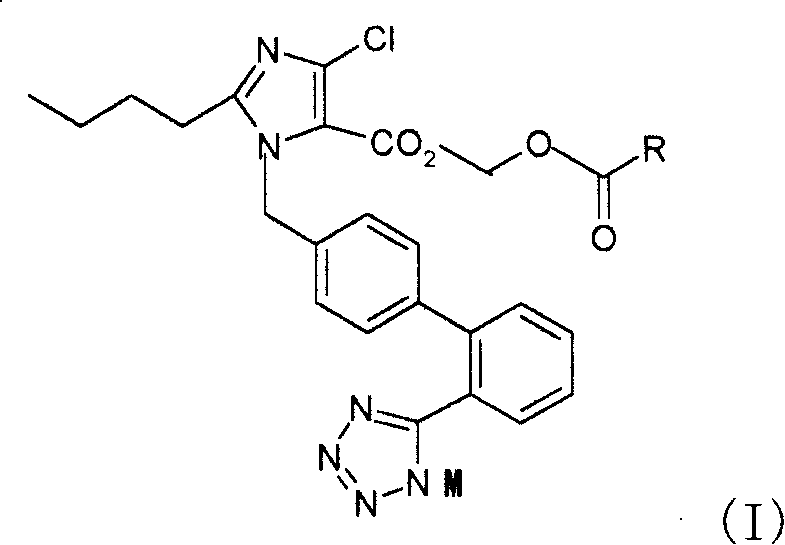
[0078] 2-Butyl-4-chloro-1-[2'-(1H-tetrazol-5-yl)1,1'-biphenyl-methyl]imidazole-5-carboxylic acid, 1-[(isopropyl) Oxy)carbonyl]methoxyester (Compound 1)
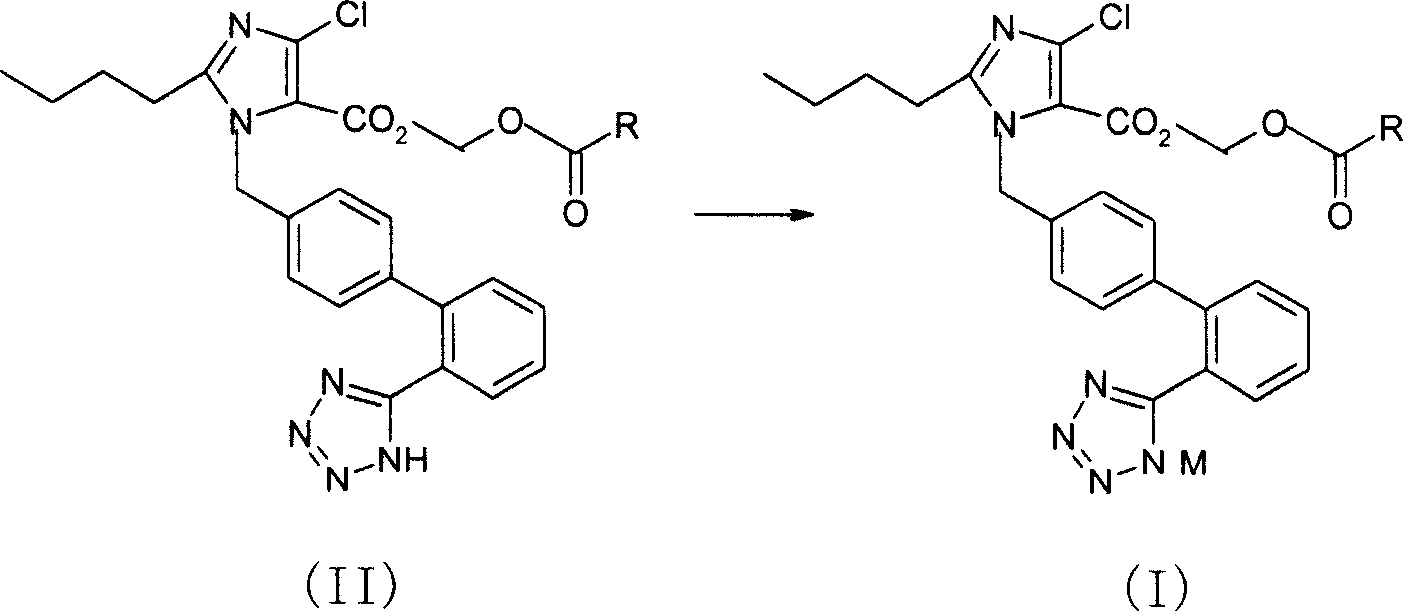
[0079] 2-butyl-4-chloro-1-[2'-(1H-tetrazol-5-yl)1,1'-biphenyl-methyl]imidazole-5-carboxylic acid (prepared by US5138069 report method) , reacted with triphenylchloromethane to give 2-butyl-4-chloro-1-[2'-(1-trityl-tetrazol-5-yl)1,1'-biphenyl-methyl yl]imidazole-5-carboxylic acid. Add 2-butyl-4-chloro-1-[2'-(1-trityl-tetrazol-5-yl)1,1'-biphenyl-methyl"imidazole to the 100ml single-necked bottle in turn -5-Carboxylic acid, 0.523g, K 2 CO 3 0.124g, 5ml of N,N-dimethylacetamide, stirred at room temperature for 20min, added 0.562g of chloromethyl isopropoxyformate at room temperature, and reacted at 45-50°C for 16h. After the reaction is completed, filter, add 30 ml of water to the filtrate, extract twice with 30 ml of ethyl acetate, dry the organic phase, and concentrate to obtain 1.724 g of an oily substance, which is dire...
Embodiment 2
[0086] 2-Butyl-4-chloro-1-[2'-(1H-tetrazol-5-yl)1,1'-biphenyl-methyl]imidazole-5-carboxylic acid, 1-[(isopropyl) Oxy)carbonyl]methoxyester, potassium salt (compound 2)
[0087]
[0088] In a 100ml three-necked flask, add 2-butyl-4-chloro-1-[2'-(1H-tetrazol-5-yl)1,1'-biphenyl-methyl]imidazole-5-carboxylic acid , 2.50 g (4.52 mmol) of 1-[(isopropoxy)carbonyl]methoxyester and 25 ml of tetrahydrofuran (THF). After stirring and dissolving, 0.645 g of potassium trimethylsiliconate (4.52 mmol, 90% content, Aldrich) dissolved in 15 ml of THF was added, and the reaction was carried out at room temperature of 28° C. for 17 h.
[0089] After the reaction, there was a small amount of white flocs in the reaction solution, which was filtered, and the filtrate was concentrated under reduced pressure to obtain a white solid crude product. Recrystallization from a mixed solution of isopropyl ether and ethanol (3:1 v / v) gave 1.42 g of 2-butyl-4-chloro-1-[2'-(1H-tetrazol-5-yl)1,1 '-Bipheny...
Embodiment 3
[0100] 2-Butyl-4-chloro-1-[2'-(1H-tetrazol-5-yl)1,1'-biphenyl-methyl]imidazole-5-carboxylic acid, 1-[(isopropyl Oxy)carbonyl]methoxy ester, potassium salt (Compound 2)
[0101]
[0102] Take 2-butyl-4-chloro-1-[2′-(1H-tetrazol-5-yl) 1,1′-biphenyl-methyl] imidazole-5-carboxylic acid, 1-[(iso Propoxy) carbonyl] methoxy ester 2.0g (3.62mmol), dissolved in 20ml isopropanol, slowly added 15% potassium 2-ethylhexanoate 4.83g (3.98mmol) at room temperature (25°C) ), heated up to 75°C, and reacted for 17h.
[0103] Heating was stopped, the temperature was naturally lowered to room temperature, and left standing for 48 hours, a small amount of white solid was precipitated. After filtration, 0.51 g of white solid was obtained, with a yield of 24%. After purification, it is 2-butyl-4-chloro-1-[2'-(1H-tetrazol-5-yl)1,1'-biphenyl-methyl]imidazole-5-carboxylic acid, 1 -[(isopropoxy)carbonyl]methoxy ester, potassium salt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com