Method of dissolubility iron salt induction photochemical degradation total fluorination substituted compound
A technology for soluble iron and compounds, applied in the field of photochemical degradation of fluorine-containing organic substances, to achieve good degradation effects, prevent pollution, and reduce the toxicity of decomposition products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]Example 1: UV (254nm) photolysis of perfluorooctanoic acid (PFOA) induced by soluble iron salts under different atmospheres
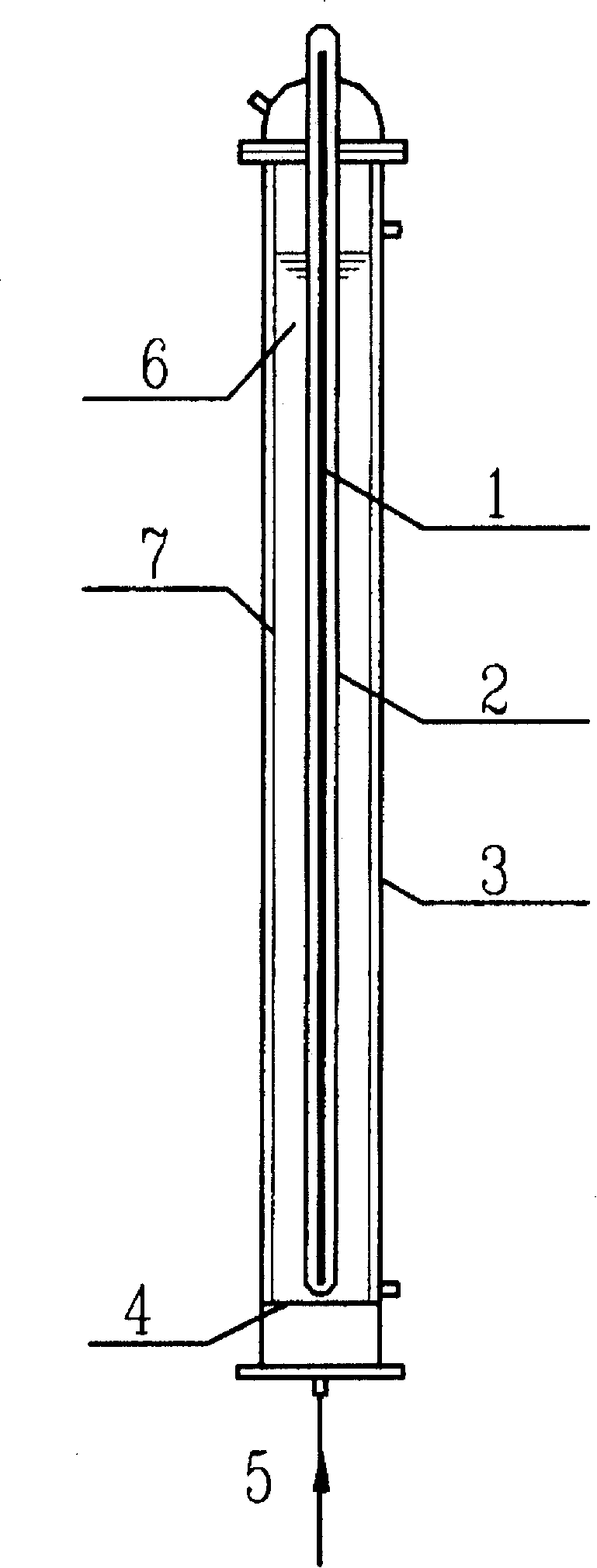
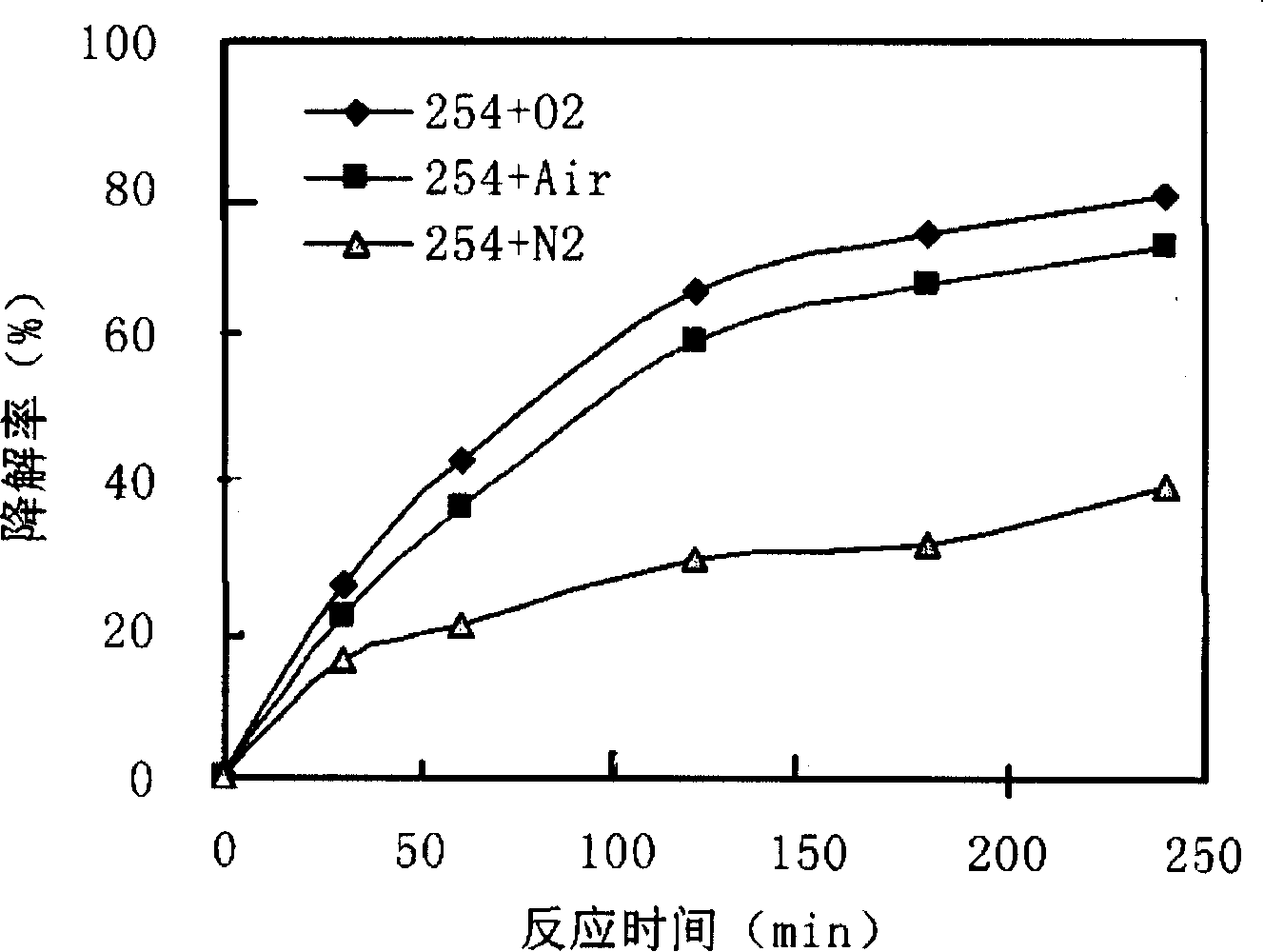
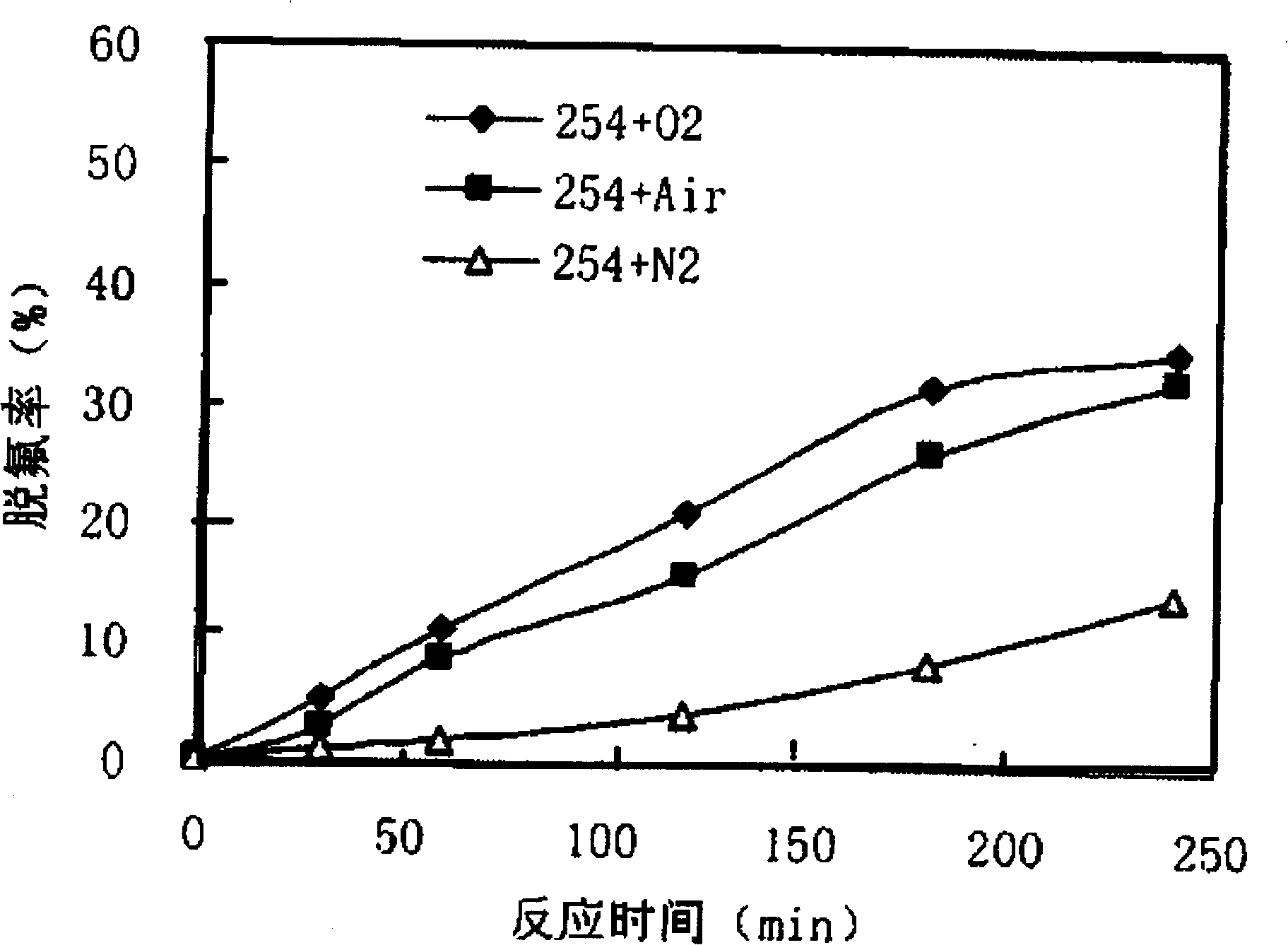
[0032] Such as figure 1 As shown, 500mL of mixed reaction solution containing PFOA (concentration of 48μM) and Fe was put into Reactor 3 2 (SO 4 ) 3 (wherein the concentration of Fe(III) is 50 μM). Oxygen enters the reactor 3 from the air inlet 5 through the gas distribution plate 4 at the bottom, and under the irradiation of a 23W ultraviolet lamp 1 with a dominant wavelength of 254nm, PFOA is decomposed and defluorinated. figure 2 , image 3 . The degradation rate is calculated according to the change of PFOA concentration in the solution, and the concentration of PFOA in the solution is analyzed by HPLC-conductivity detector. The defluorination rate is calculated according to the change value of fluoride ion concentration in water during the reaction process, and the fluoride ion concentration in water is measured by ion chromatography (...
Embodiment 2
[0033] Example 2: UV (254nm) photolysis of perfluorooctanoic acid (PFOA) induced by different concentrations of soluble iron salts under an oxygen atmosphere
[0034] Such as figure 1 As shown, 500mL of the mixed reaction solution containing PFOA (concentration of 48μM) and Fe 2 (SO 4 ) 3 (wherein the Fe(III) concentration is 0-80 μM). Oxygen enters the reactor 3 from the air inlet 5 through the gas distribution plate 4 at the bottom, and under the irradiation of a 23W ultraviolet lamp 1 with a dominant wavelength of 254nm, PFOA is decomposed and defluorinated. Figure 4 , Figure 5 . The degradation rate is calculated according to the change of PFOA concentration in the solution, and the concentration of PFOA in the solution is analyzed by HPLC-conductivity detector. The defluorination rate is calculated according to the change value of fluoride ion concentration in water during the reaction process, and the fluoride ion concentration in water is measured by ion chromat...
Embodiment 3
[0035] Example 3: Perfluorooctane sulfonic acid (PFOS) UV (254nm) photolysis induced by soluble iron salts
[0036] Such as figure 1 As shown, 500mL mixed reaction solution containing PFOS (concentration of 40μM) and Fe(NO 3 ) 3 (where the Fe(III) concentration is 30 μM). Oxygen enters the reactor 3 from the air inlet 5 through the gas distribution plate 4 at the bottom, and under the irradiation of a 23W ultraviolet lamp 1 with a dominant wavelength of 254nm, the decomposition effect of PFOS can be seen in Figure 4 . The degradation rate is calculated according to the change of PFOS concentration in the solution, and the concentration of PFOS in the solution is analyzed by HPLC-conductivity detector. After 240 minutes of reaction, 23% of PFOS was degraded.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com