Methods of suppressing UV light-induced skin carcinogenesis
A technology for ultraviolet light and skin cancer, which is used in pharmaceutical formulations, cosmetic preparations, cosmetic preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Example 1. Preparation of sulforaphane from broccoli sprouts
[0047]Seeds of cauliflower (Brassica oleracea italica, cv. DeCicco) certified to have not been treated with any pesticides or other seed treatment chemicals were germinated and treated as described by Fahey et al. (12). Briefly, seeds were surface disinfected with a 25% aqueous solution of Clorox(R) bleach containing a trace amount of ALconox(R) detergent and rinsed thoroughly with water. The seeds were then spread in a single layer in inclined perforated plastic trays and misted with filtered water for 30 seconds, about 6 times per hour, with fluorescent lighting on. After 3 days, the sprouts were directly dropped into the boiling water in the steam-jacketed pot to make it stop growing, and the steam-jacketed pot was boiled again and stirred for about 5 minutes. This treatment inactivates endogenous myrosinase in shoots and extracts glucosinolates. The precursor glucoraphanin of sulforaphane was found to be the ...
Embodiment 2
Example 2. Treatment of Keratinocytes with Sulforaphane
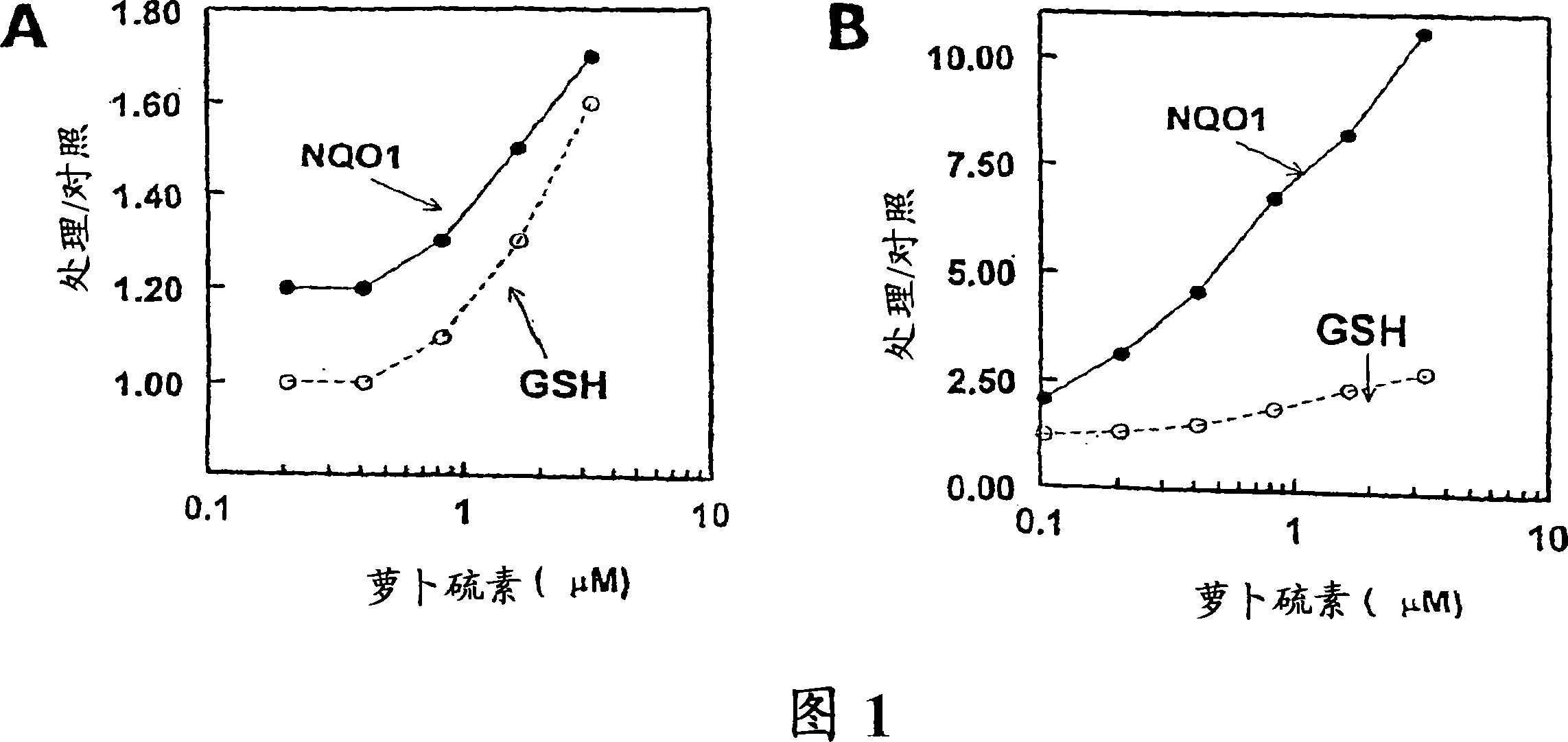
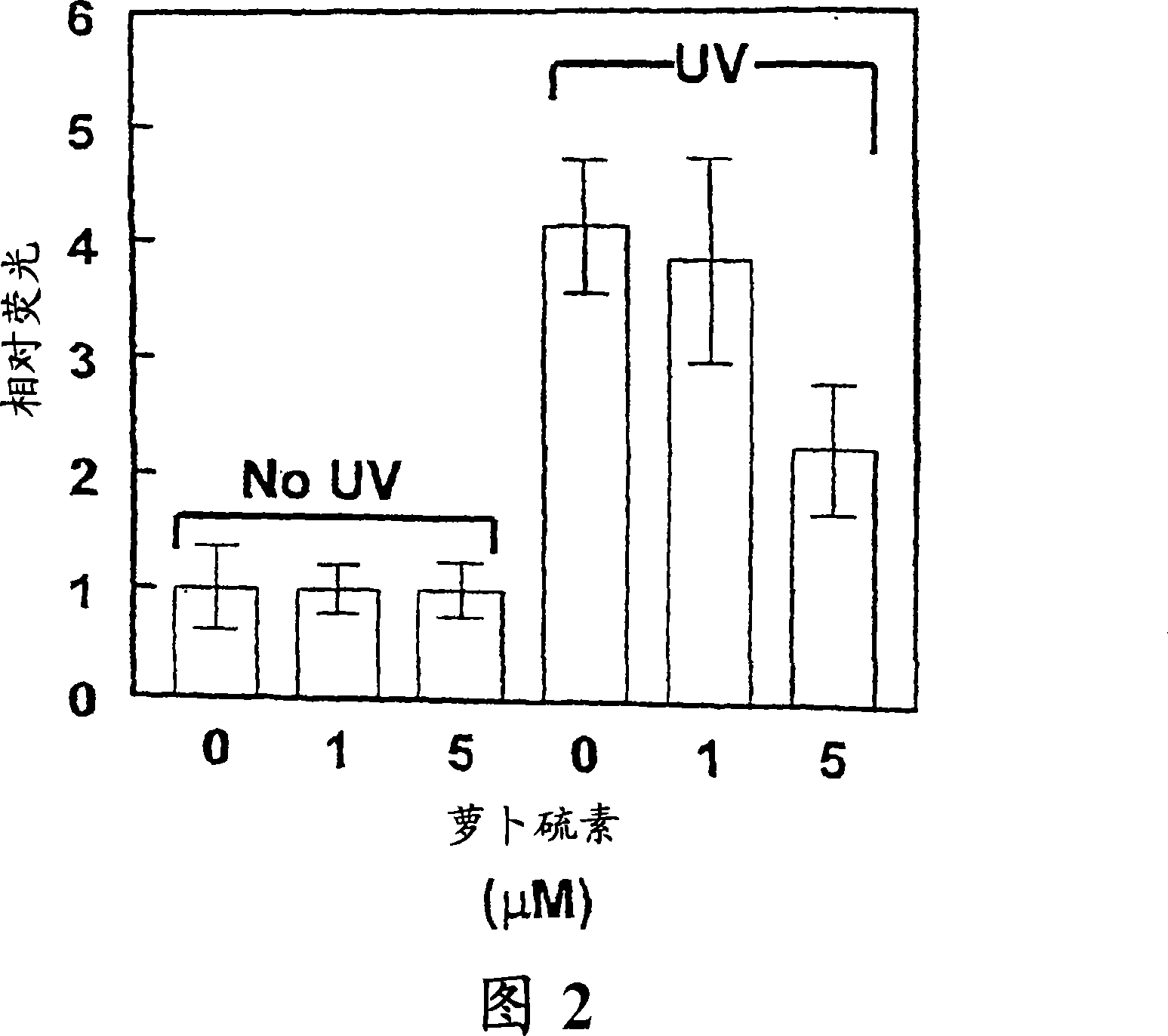
[0048] Glutathione is the major and most abundant cellular nonprotein thiol and constitutes a key part of cellular defense: it readily reacts with potentially harmful electrophiles, participates in reactivity by scavenging free radicals and reducing peroxides Detoxification of oxygen intermediates and their toxic metabolites. The ability to increase cellular GSH levels is critical to combat oxidative stress. To this end, we investigated the ability of sulforaphane-induced phase 2 responses against UVA-induced oxidative stress in keratinocyte cultures. UVA was chosen for this study because its genotoxicity is thought to be mainly due to the generation of reactive oxygen intermediates.
cell culture
[0049] HaCaT human keratinocytes (gifted by G.Tim Bowden, Arizona Cancer Center, Tucson) were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 5% FBS; PE mouse keratinocytes (Stuart H.Yuspa, National...
Embodiment 3
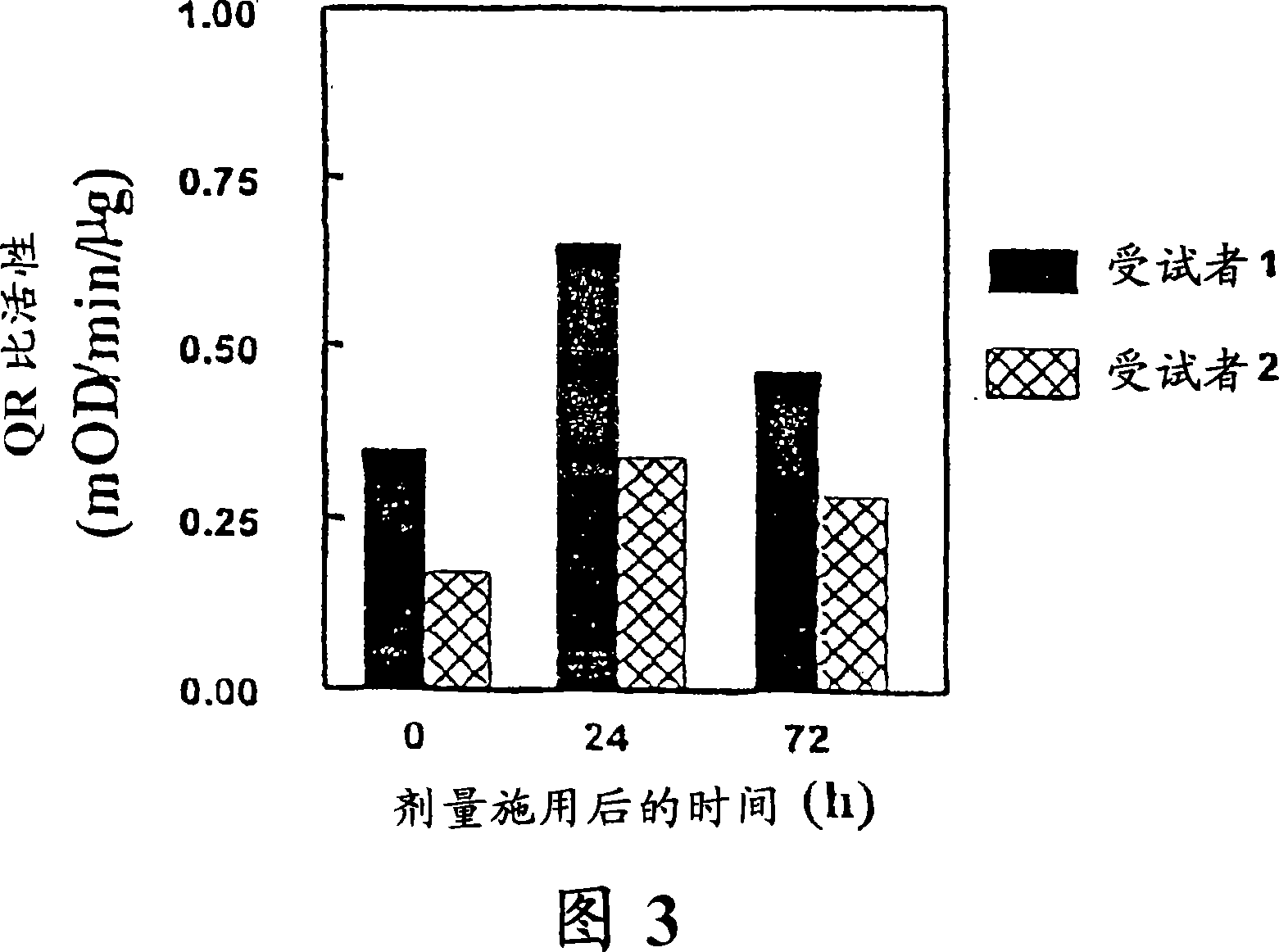
Example 3. Effect of Topical Administration of Sulforaphane on NQO1 and GSH in Mice
[0053] Phase 2 responses were next assessed in vivo in SKH-I hairless mice. Female SKH-I hairless mice (4 weeks old) were obtained from Charles River Breeding Laboratories (Wilmington, MA) and acclimatized in our animal facility for 2 weeks prior to the start of experiments. Animals were housed on a 12 hr light / 12 hr dark cycle, 35% humidity, with free access to water and pelleted AIN76A diet (Harlan TekLad, no inducer). All animal experiments were in accordance with National Institutes of Health Guidelines and were approved by the Johns Hopkins University Animal Care and Use Committee.
[0054] With 100 μl standardized myrosinase-hydrolyzed broccoli sprout extract (containing 1 μmol sulforaphane) or medium (100 μl 80% acetone: 20% water, v / v), 7-week-old SKH-I hairless Rats (5 per group) were treated locally on their backs. Animals were euthanized 24 hours later, and the dorsal skin was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com