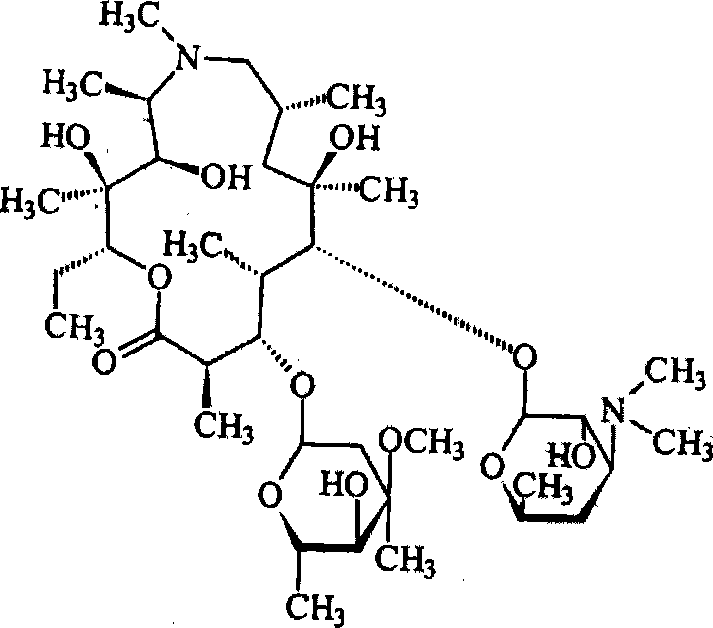
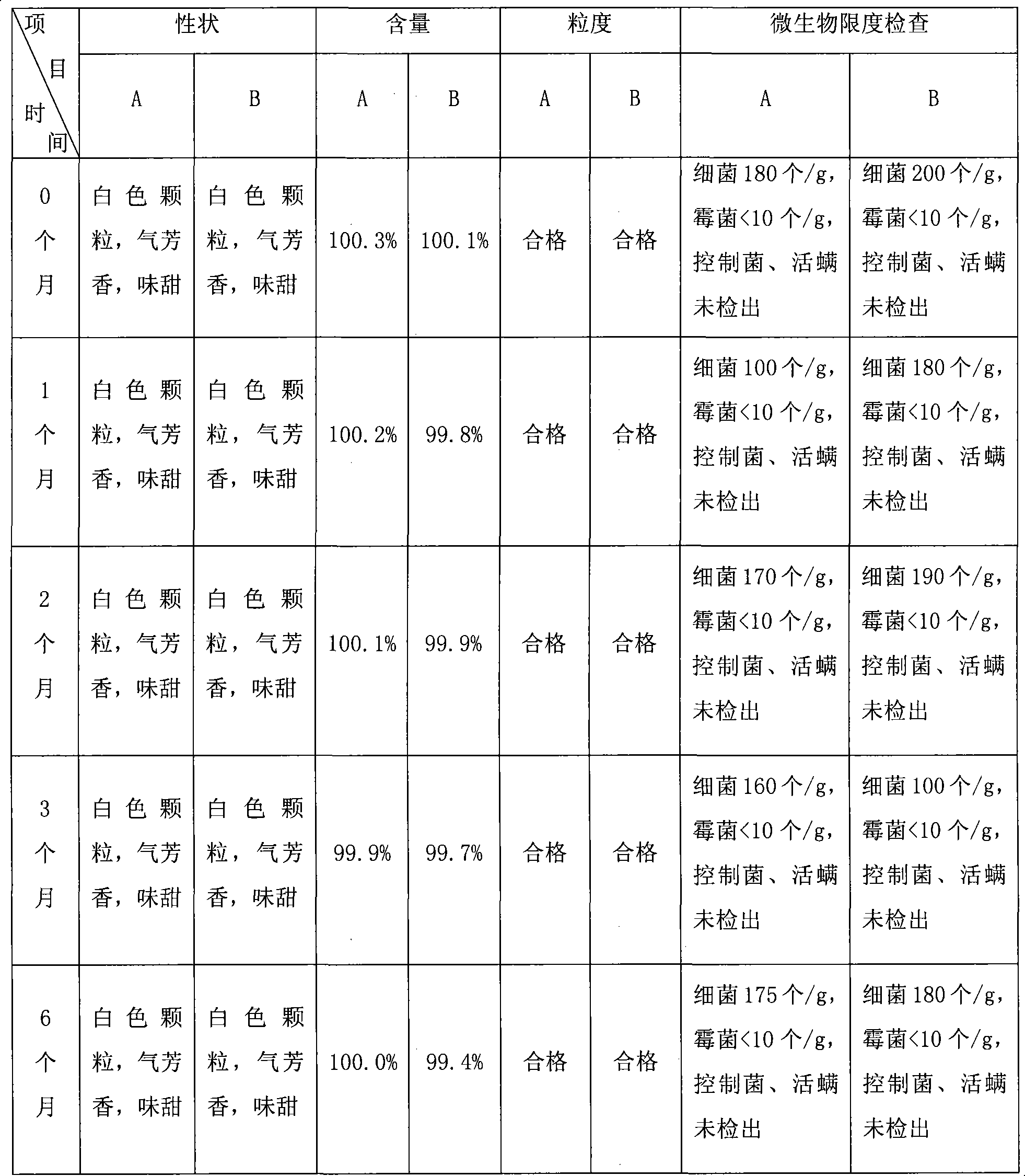
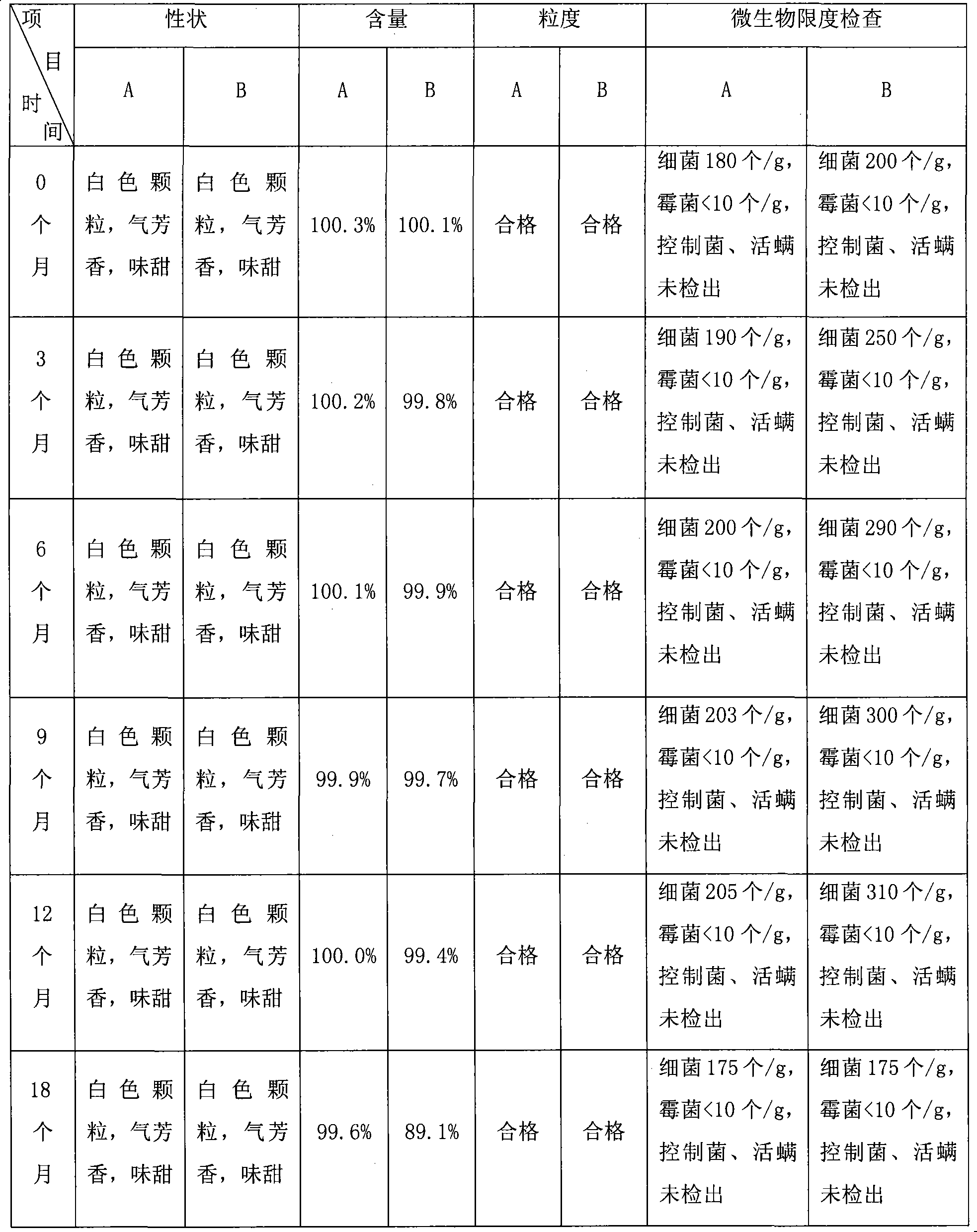
Azithromycin mix suspension grain and method for preparing the same
A technology of azithromycin and suspension granules, applied in the field of medicine, can solve the problems of azithromycin suspension granules without teaching or hinting, without providing, without providing suspension uniformity and stability of azithromycin suspension granules, etc. Achieve the effect of improving moisture absorption and good formability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 1. Specification: 0.1g
[0075] 2. Prescription:
[0076] Azithromycin 100g
[0077] Sucrose 500g
[0078] Starch 60g
[0079] Mannitol 16g
[0080] Stevia 20g
[0081] 95% ethanol appropriate amount
[0082] Coconut Flavor Appropriate amount
[0083]
[0084] Make 1000 bags
[0085] 3. Preparation process:
[0086] (1) Take the raw materials and auxiliary materials of the prescription amount, pulverize azithromycin, sucrose and mannitol respectively, pass through a 100 mesh sieve, and pass the starch through a 120 mesh sieve to obtain the original and auxiliary material powders;
[0087] (2) Place the raw and auxiliary material powder obtained in the previous step in a mixer and dry-mix for 10 minutes, add 95% ethanol, and wet-mix for 30 seconds to make a soft material. Granules to obtain wet granules;
[0088](3) Place the wet granules prepared in the previous step evenly in the fluidized dryer, adjust the air inlet te...
Embodiment 2
[0091] 1. Specification: 0.25g
[0092] 2. Prescription:
[0093] Azithromycin 250g
[0094] Sucrose 1250g
[0095] Starch 150g
[0096] Mannitol 40g
[0097] Stevia 50g
[0098] 95% ethanol appropriate amount
[0099] Coconut Flavor Appropriate amount
[0100]
[0101] Make 1000 bags
[0102] 3. Preparation process
[0103] (1) Take the raw materials and auxiliary materials of the prescription amount, pulverize azithromycin, sucrose and mannitol respectively, pass through a 100 mesh sieve, and pass the starch through a 120 mesh sieve to obtain the original and auxiliary material powders;
[0104] (2) Put the raw and auxiliary material powder obtained in the previous step into a mixer, seal and dry mix for 5 minutes, add 95% ethanol, wet mix for 150 seconds to make a soft material, and granulate under the conditions of an ambient temperature of 20°C and a relative humidity of 25%. , to get wet particles;
[0105] (3) Place the w...
Embodiment 3
[0108] 1. Prescription:
[0109] Azithromycin 200g
[0110] Sucrose 1000g
[0111] Starch 120g
[0112] Mannitol 32g
[0113] Stevia 40g
[0114] 95% ethanol appropriate amount
[0115] Coconut Flavor Appropriate amount
[0116]
[0117] Make 1000 bags
[0118] 2. Preparation process:
[0119] (1) Take the raw materials and auxiliary materials of the prescription amount, pulverize azithromycin, sucrose and mannitol respectively, pass through a 100 mesh sieve, and pass the starch through a 120 mesh sieve to obtain the original and auxiliary material powders;
[0120] (2) Put the raw and auxiliary material powder obtained in the previous step into a mixer, seal and dry mix for 15 minutes, add 95% ethanol, and wet mix for 100 seconds to make a soft material, and granulate to obtain wet granules;
[0121] (3) Place the wet granules prepared in the previous step evenly in the fluidized dryer, adjust the air inlet temperature to 65°C, dry fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com