Medicine use of interleukin-22
A technology of interleukin and its application, applied in the field of medical application of interleukin-22
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1 human and mouse interleukin-22 gene cloning
[0072] Cloning of human interleukin-22 gene: Take normal human peripheral blood mononuclear cells and use anti-CD 3 mAb stimulation and incubation overnight. The method of extracting tRNA was ultracentrifugation. cDNA was synthesized by using dT as a primer. Primers for human interleukin-22 gene include sense strand (5'-GCAGAATCTGAACAGGTTC-3') and antisense strand (5'-GGCATCTAATTGTTATTTTCTAG-3') for PCR amplification. The amplified DNA of human interleukin-22 was cloned into the expression vector of Escherichia coli.
[0073] Cloning of mouse interleukin-22 gene: C57BL / 6 female mice were injected with LPS (5 mg / kg, sc), and the spleen was taken 20 hours later to extract RNA. cDNA was synthesized by using dT primer. The primers for mouse interleukin-22 include sense strand (5'-CTCTCACTTATCAACTGTTGAC-3') and antisense strand primers (5'-GATGATGGACGTTAGCTTCTCAC-3'), which are cloned into the Escherichia coli e...
Embodiment 2
[0075] Example 2 Expression of human and mouse interleukin-22 proteins
[0076] Escherichia coli BL21 (E.Coli) was used to express the recombinant protein. E. coli were destroyed with a homogenate under high pressure. Inclusion bodies containing interleukin-22 were obtained by centrifugation. The inclusion bodies were fully washed in a buffer solution (containing 50 mM Tris-HCl pH 8.0, NaCl 100 mM, EDTA 1 mM, DTT 1 mM, Sodium Deoxycholate 0.5%). Inclusion bodies were further dissolved in 8M urea, 50 mM Mes, 10 mM EDTA and 0.1 mM DTT, pH 6.5. The dissolved inclusion bodies were reduced and reset four times in 20 hours in 100mM Tris HCL, 2mM EDTA, 0.5m L-arginine, 1mM reduced glutathione, 0.1mM oxidized glutathione, pH8.0. It was then concentrated and further purified by column chromatography on Superdex75 (Amersham). The recombined protein was eluted with Tris-HCl 20mM, NaCl 50mM pH 7.0. The purity of interleukin-22 was stained with SD3-PAGE gel (purity>95%). And quantita...
Embodiment 3
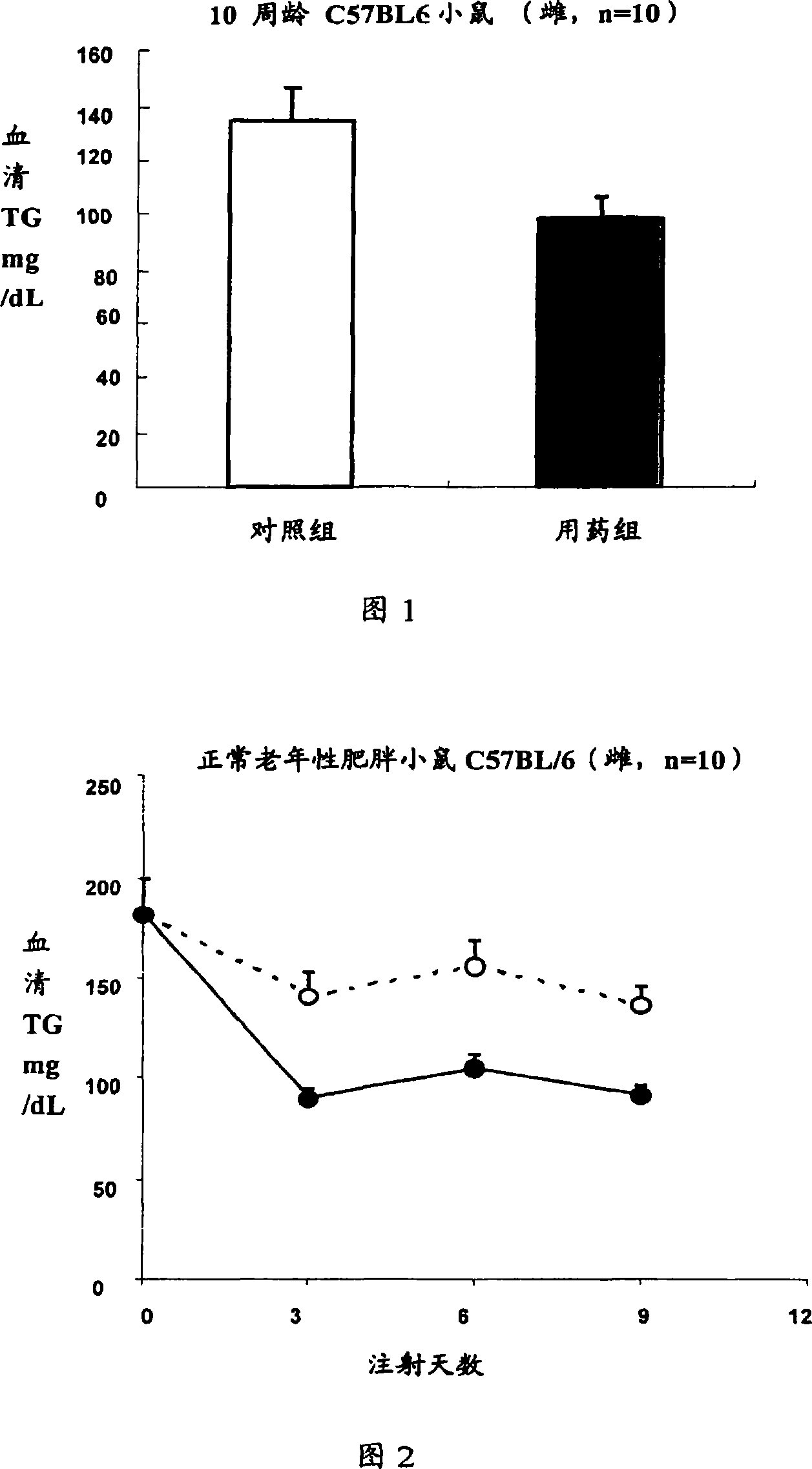
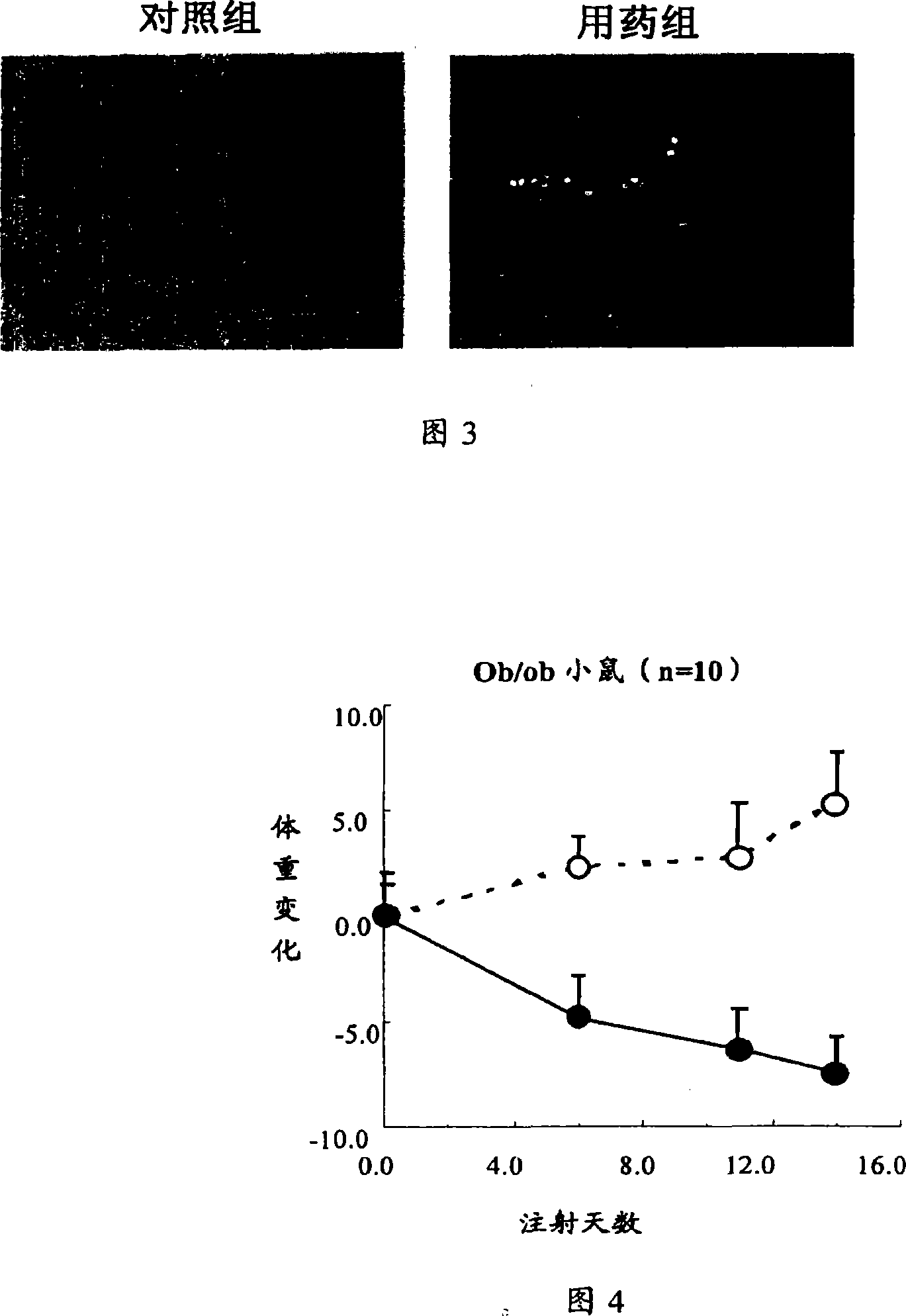
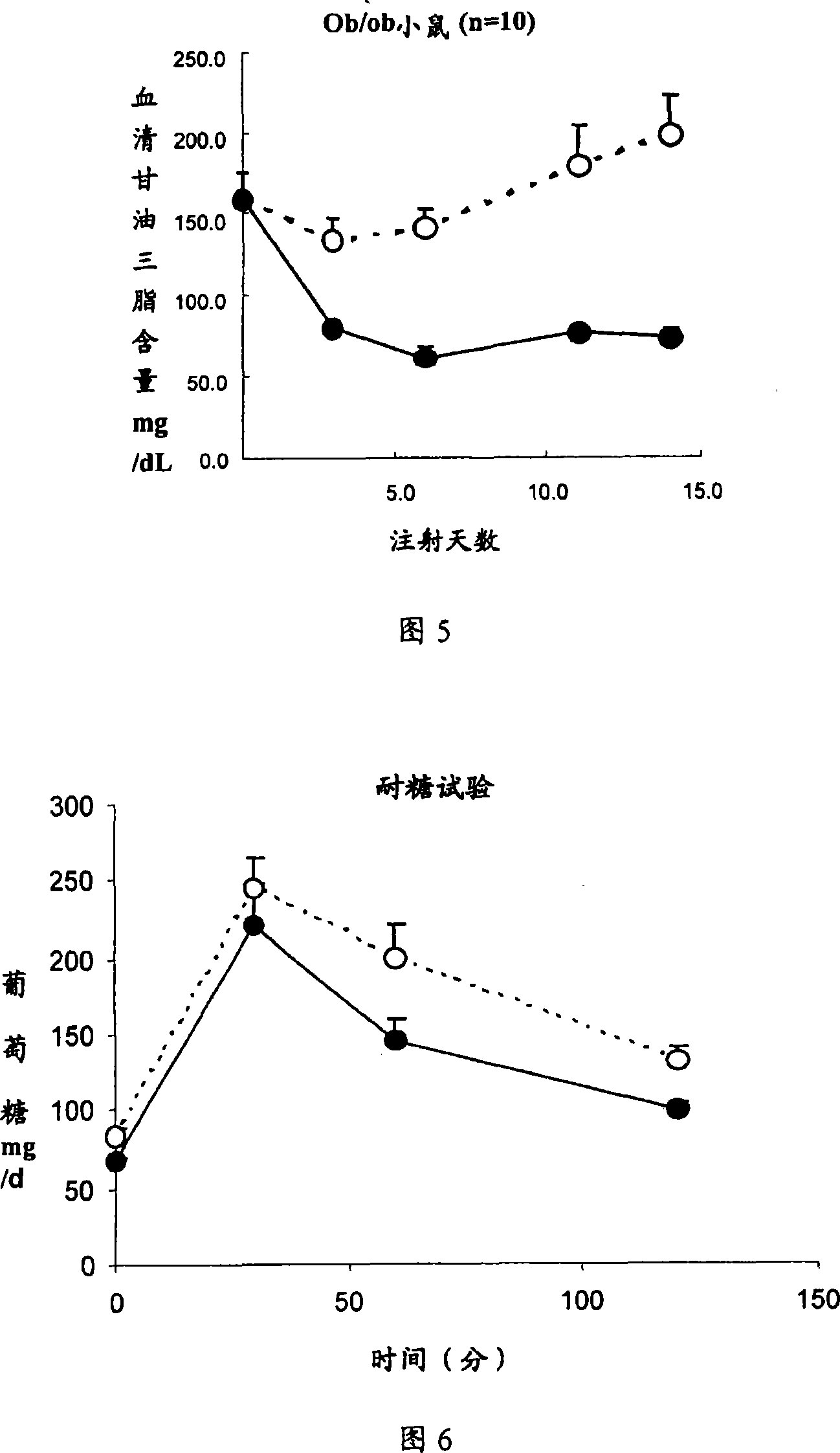
[0077] Example 3 Recombinant interleukin-22 reduces serum total triglyceride (TG) levels in normal mice
[0078] Normal mice: C56BL / 6, female, 8-12 weeks old, weighing 20-25 grams (n=10). Recombinant mouse interleukin-22 protein was injected subcutaneously every day, the concentration range: 0, 3, 30, 100, 300ug / kg / d. Interleukin-22 was dissolved in 0.1% BSA, Bovine Serum Albumin, PBS, Phosphate Buffered Saline, pH7.0, and injected once a day for 7 consecutive days. The control group was injected with the same volume of solvent: 0.1% BSA, Bovine Serum Albumin, PBS, Phosphate Buffered Saline, pH7.0.
[0079] 100ul of blood was taken from the eye socket at different times after the drug treatment, the blood was allowed to coagulate, the serum was taken and diluted with normal saline, and the content of total triglyceride was measured on an automatic biochemical analyzer.
[0080] The results showed that recombinant mouse interleukin-22 could significantly reduce the concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com