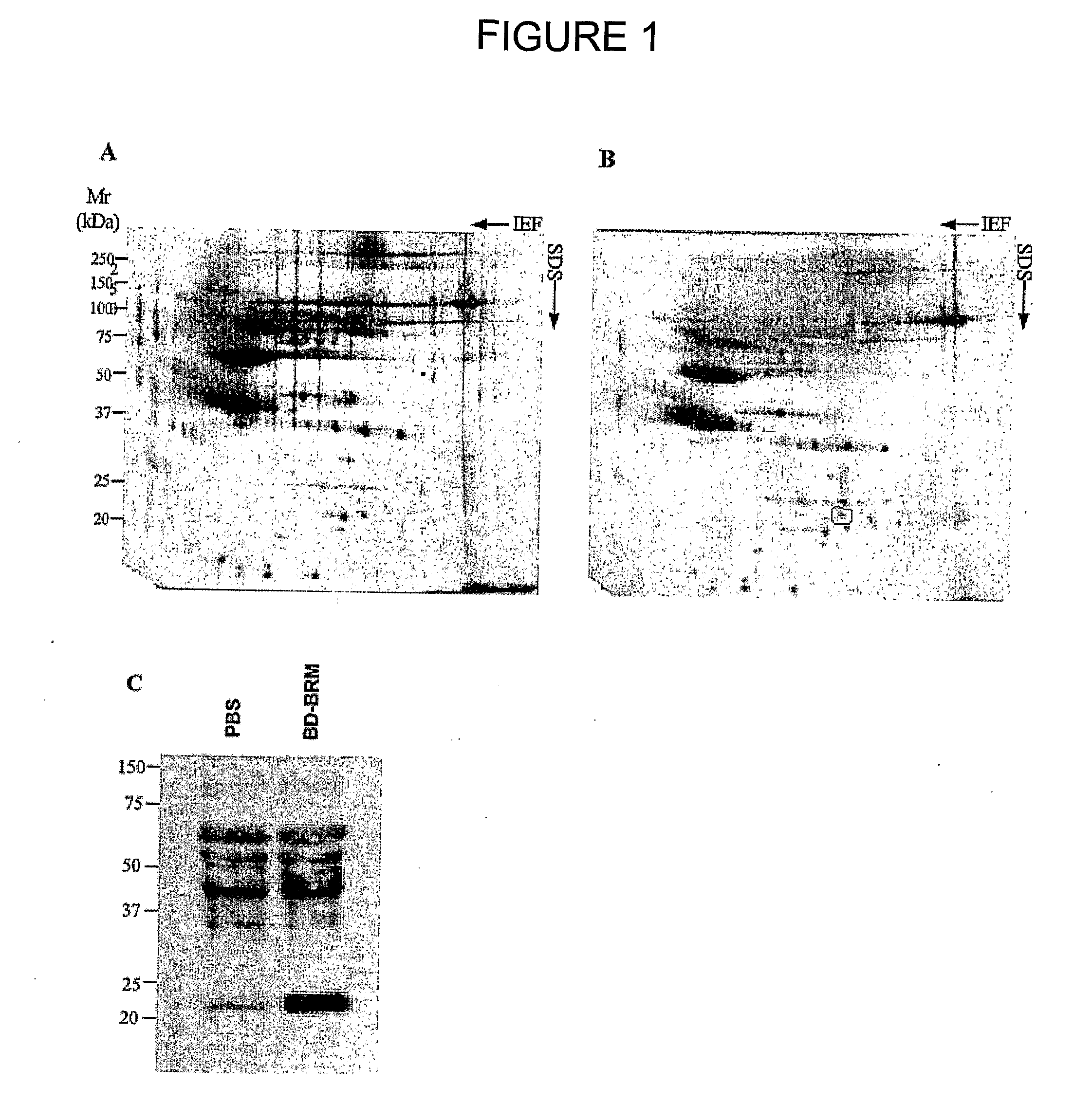
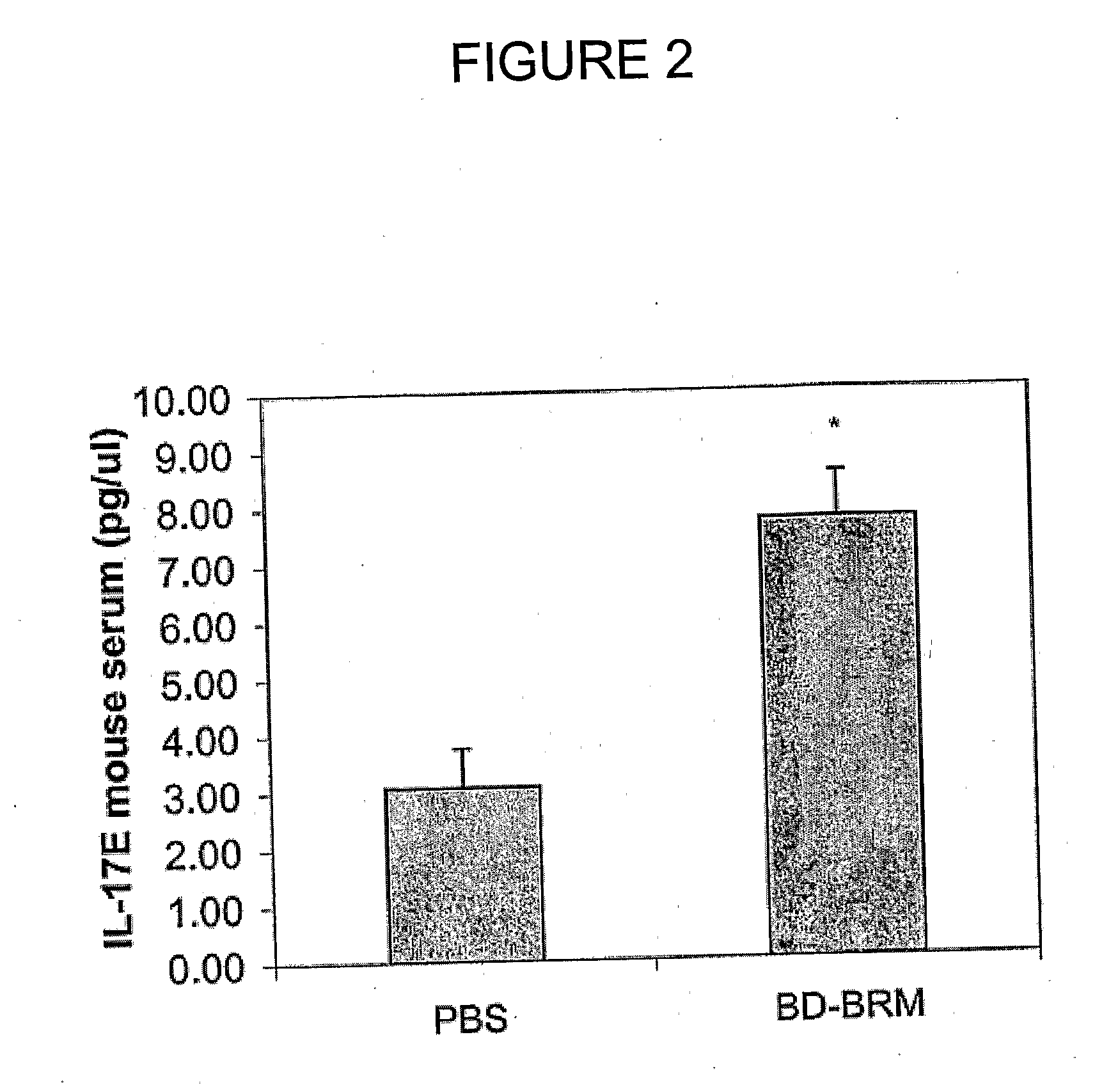
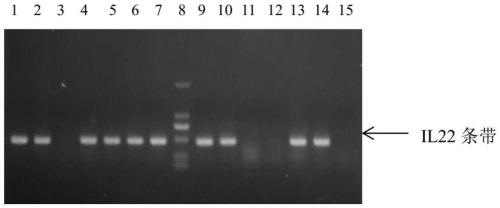
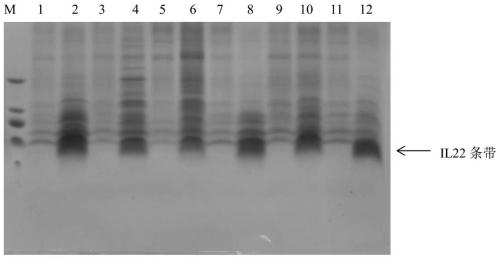
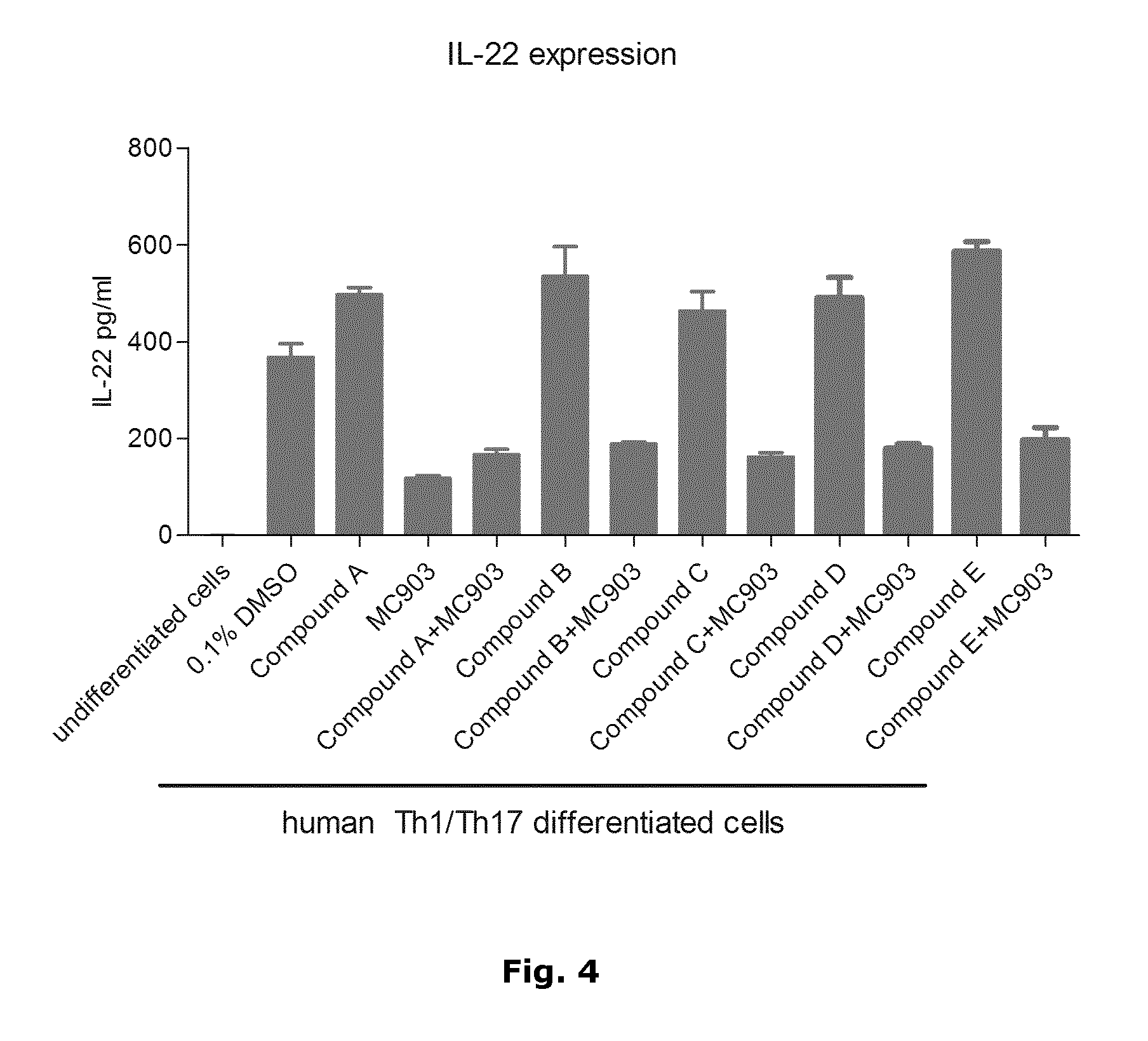
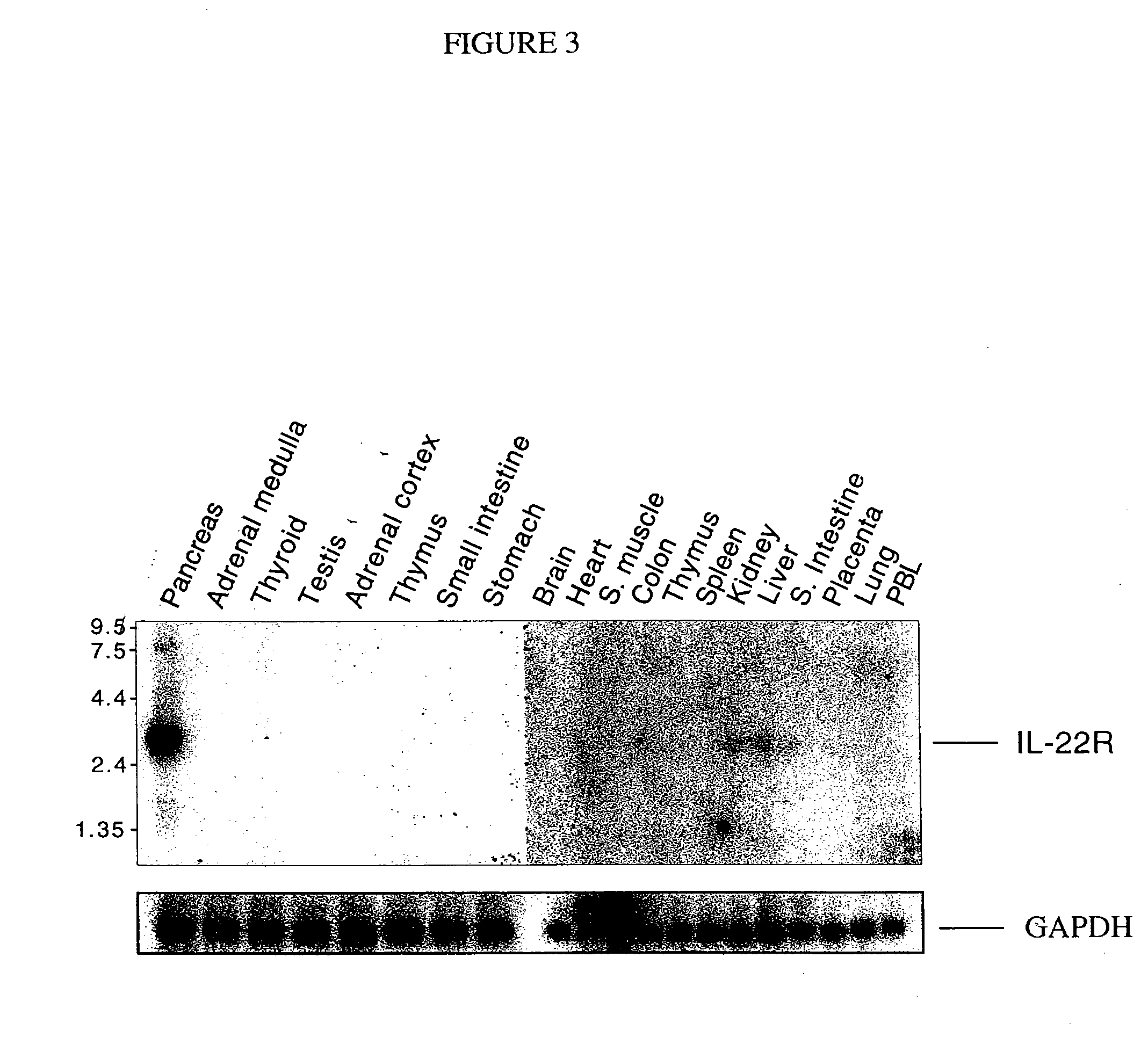
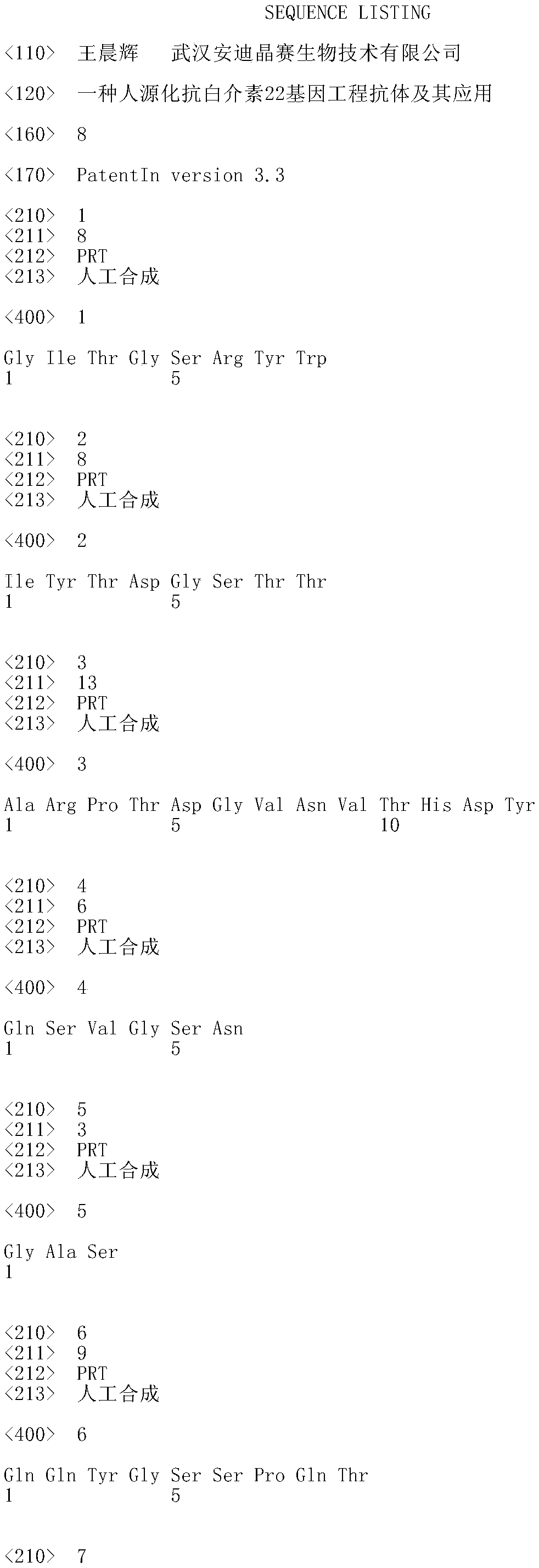Patents
Literature
37 results about "Interleukin 22" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interleukin-22 (IL-22) is protein that in humans is encoded by the IL22 gene.
Human interleukins-22 mutant as well as construction method and use thereof
InactiveCN101225110AIncrease productionLow costRecombinant DNA-technologyFermentationMutated proteinWhite blood cell
The invention discloses a receptor binding site of the interleukin-22, forming a basis for the utilization of the interleukin-22; the invention also discloses a mutant protein where the binding activity for the 67th and 71st Asp to change into Ala is improved, and the mutant protein can play a more significant role even than the interleukin-22. The receptor binding site of the interleukin-22 also provides a construction method for the interleukin-22 mutant, and the construction method has the advantages of high yield, low cost, and applicability to production and application in large scales.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Interleukin-22 polypeptides, nucleic acids encoding the same and methods for the treatment of pancreatic disorders
The present invention is directed to interleukin-22 polypeptides and nucleic acid molecules encoding those polypeptides. Also provided herein are vectors and host cells comprising those nucleic acid sequences, chimeric polypeptide molecules comprising the polypeptides of the present invention fused to heterologous polypeptide sequences, antibodies which bind to the polypeptides of the present invention and to methods for producing the polypeptides of the present invention.
Owner:GENENTECH INC
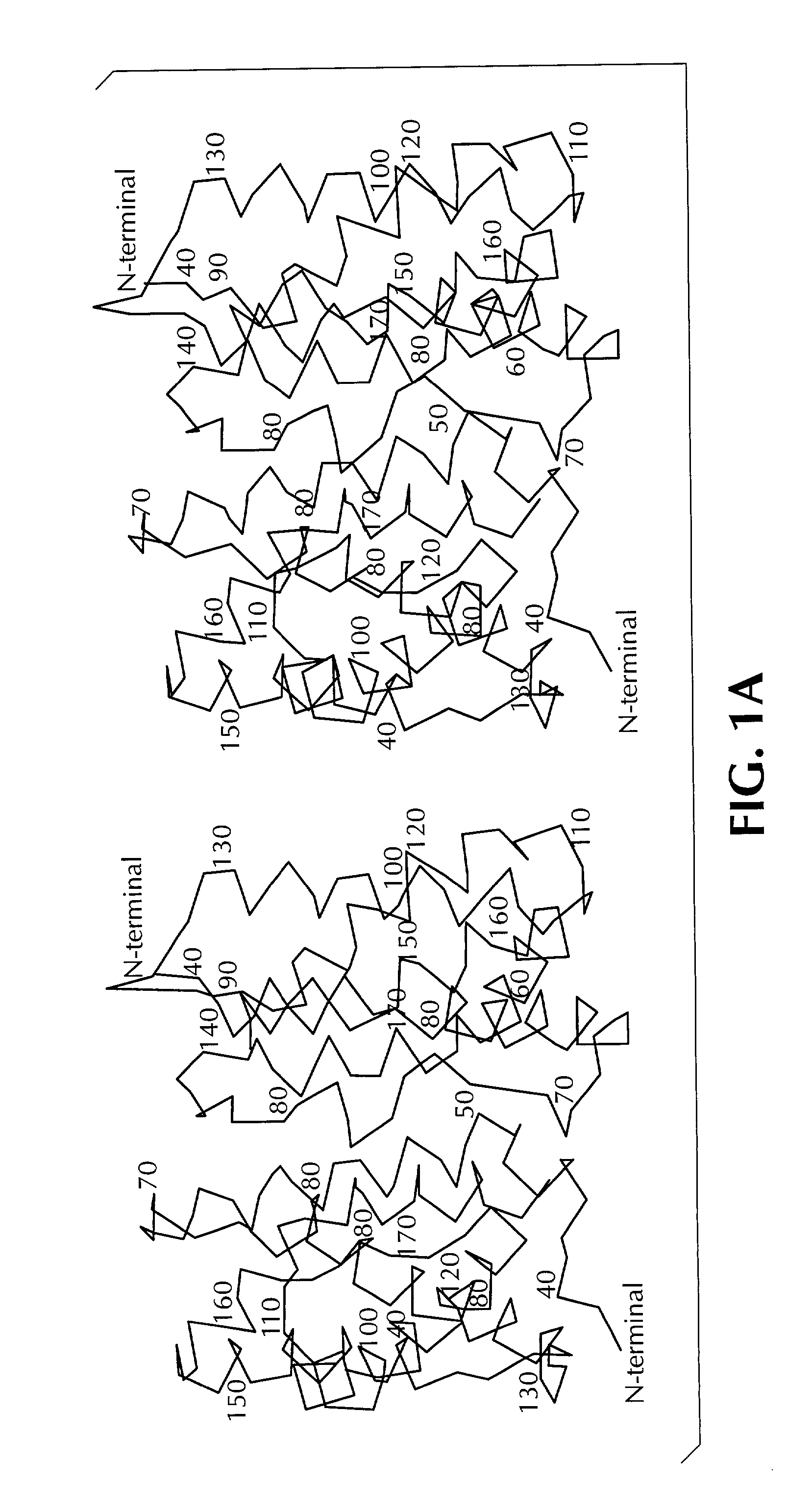
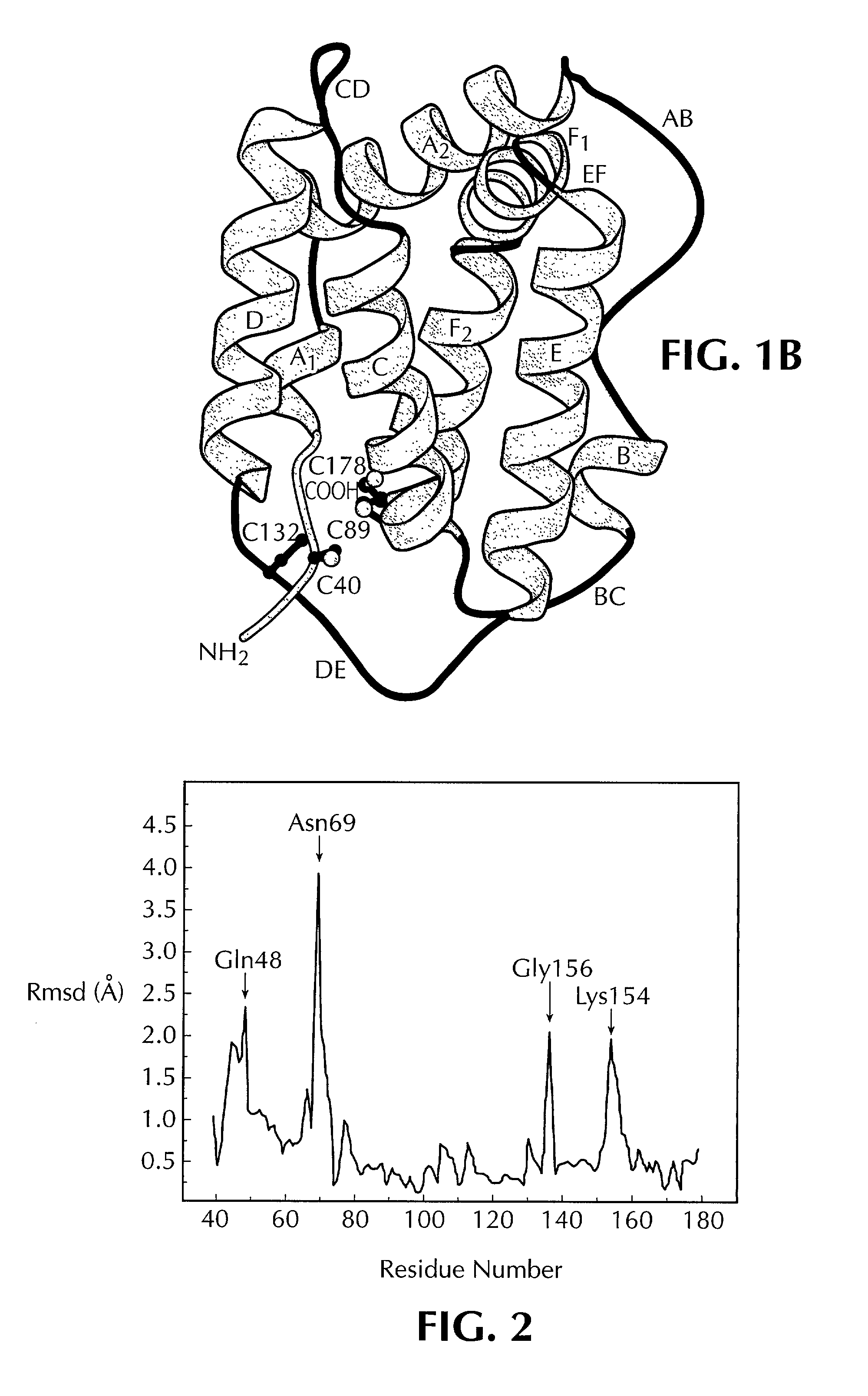
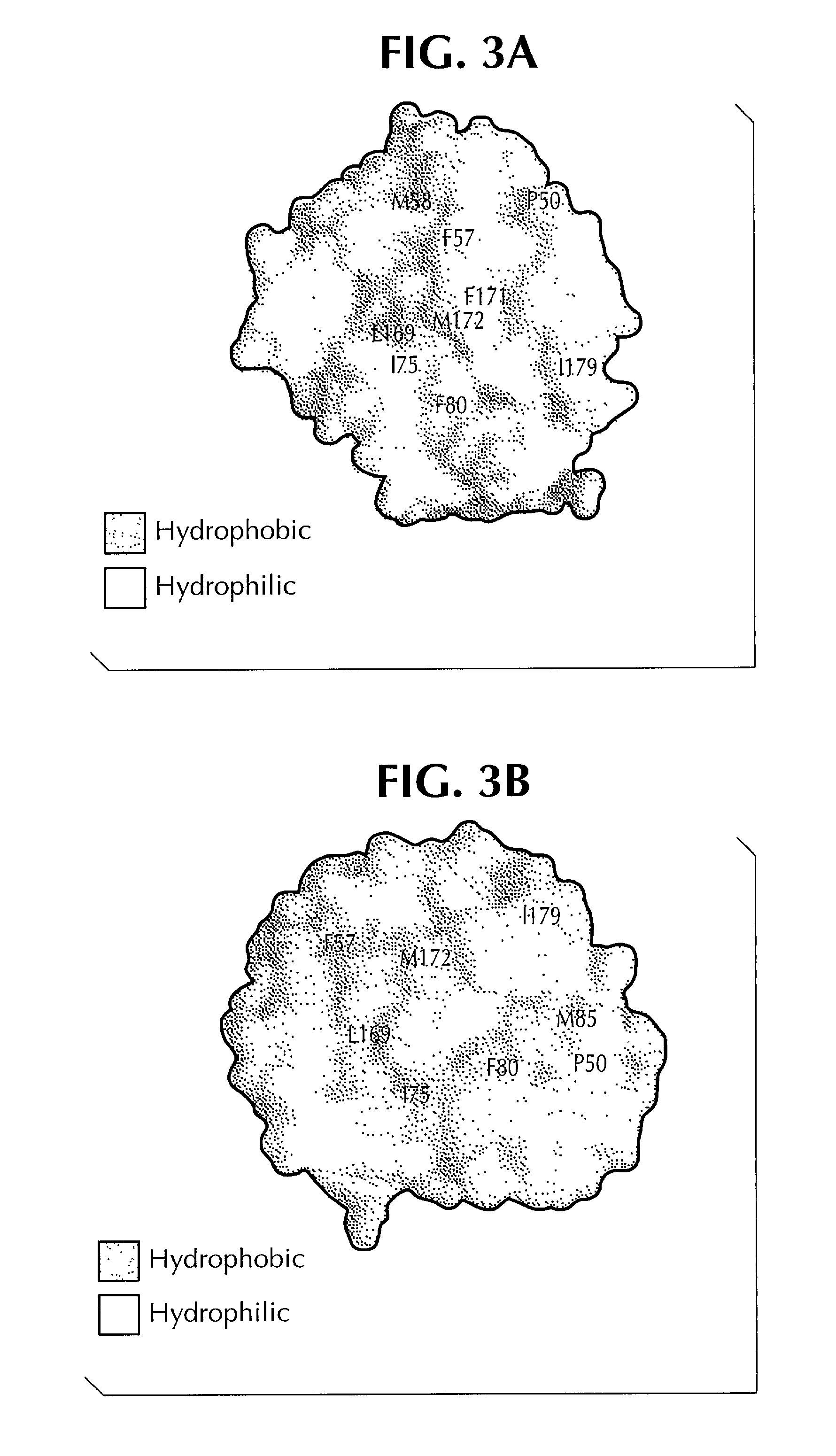
Crystal structure of human interleukin-22
InactiveUS20020187512A1Increased glycosylationPrevents and reduces dimerizationBiological testingFermentationWhite blood cellCrystal structure
This invention provides the three dimensional structure of human IL-22 and recombinant human IL-22 with mutations in the receptor binding regions and the dimerization interface and nucleic acid molecule encoding same. This invention also relates to methods of using pharmaceutical formulations and mimetics of the recombinant IL-22 and to methods for generating mutants based on the crystalline structure of IL-22.
Owner:LUDWIG INST FOR CANCER RES
Use of interleukin-22 in the treatment of fatty liver disease
InactiveUS20140377222A1Lower Level RequirementsReduces Free Fatty AcidPeptide/protein ingredientsMetabolism disorderFatty liverWhite blood cell
The present invention relates to use of interleukin-22 (IL-22) for treating fatty liver disease by decreasing the levels of transaminases. The use of IL-22 in decreasing the levels of transaminases is also provided.
Owner:GENERON (SHANGHAI) CORP LTD
Uses of interleukin-22(il-22) in treating and preventing nerve damage diseases or neurodegenerative diseases
ActiveUS20150147293A1Enabling recoveryFunction increaseNervous disorderPeptide/protein ingredientsIn vivoDisease cause
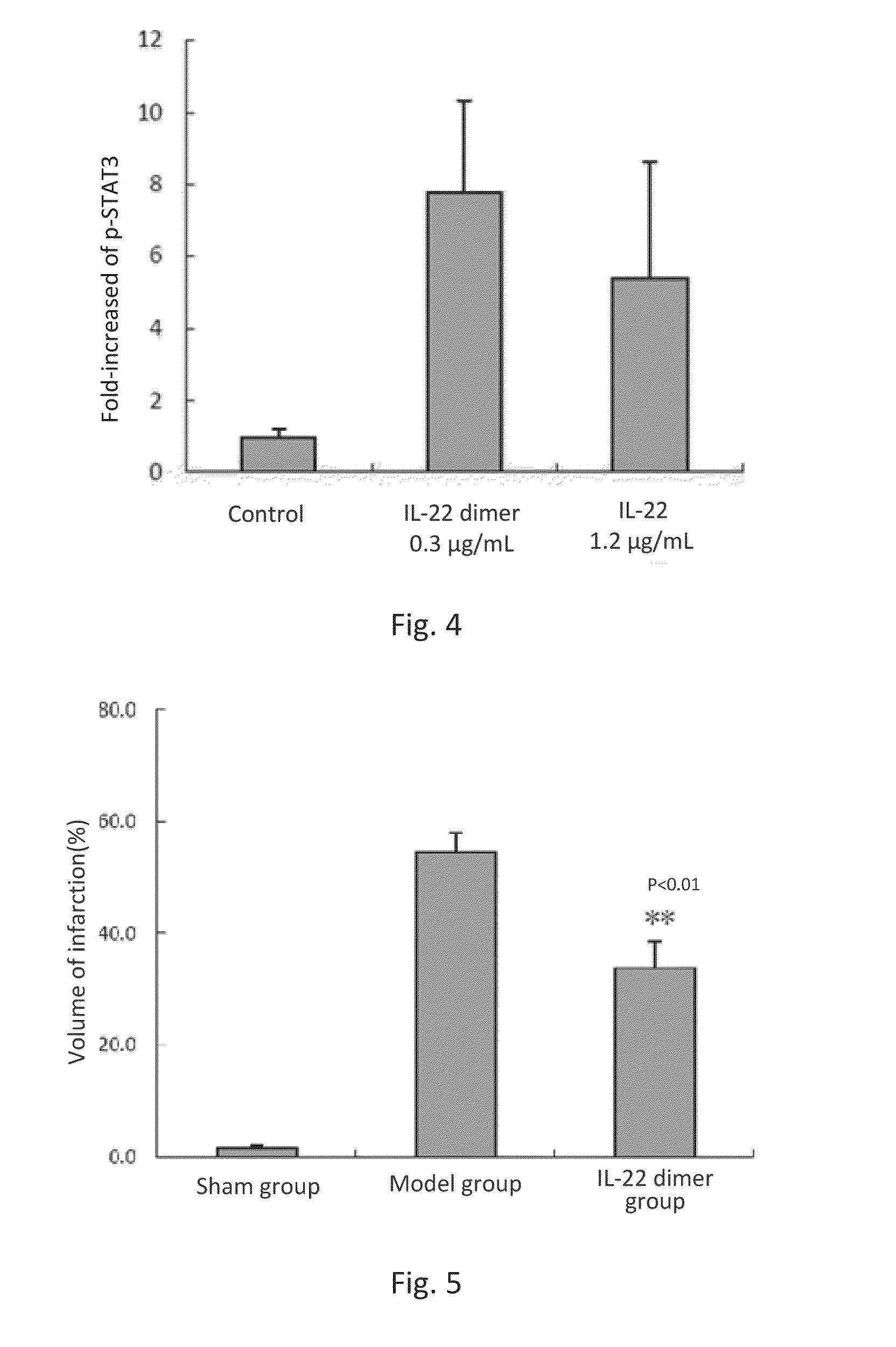
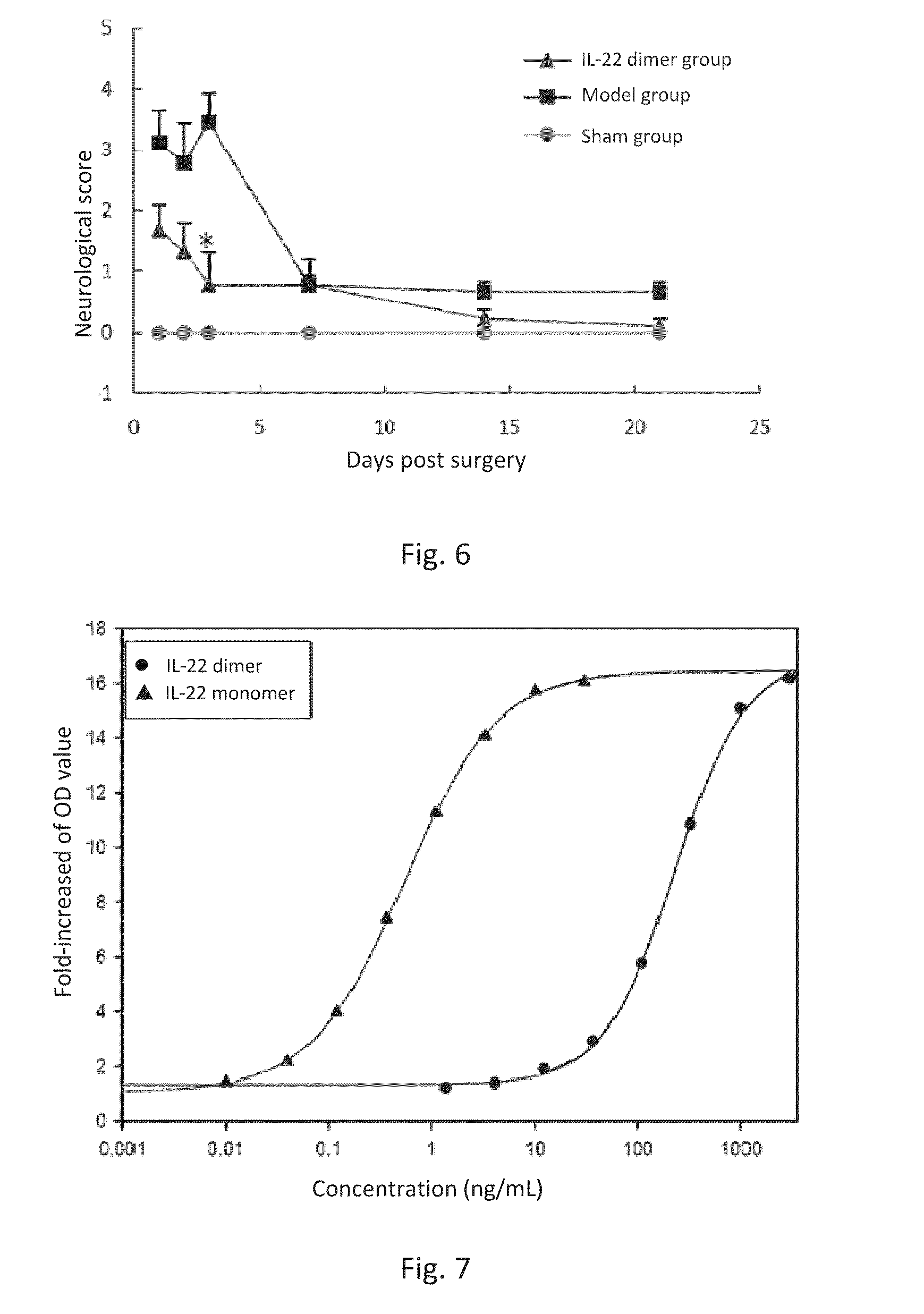
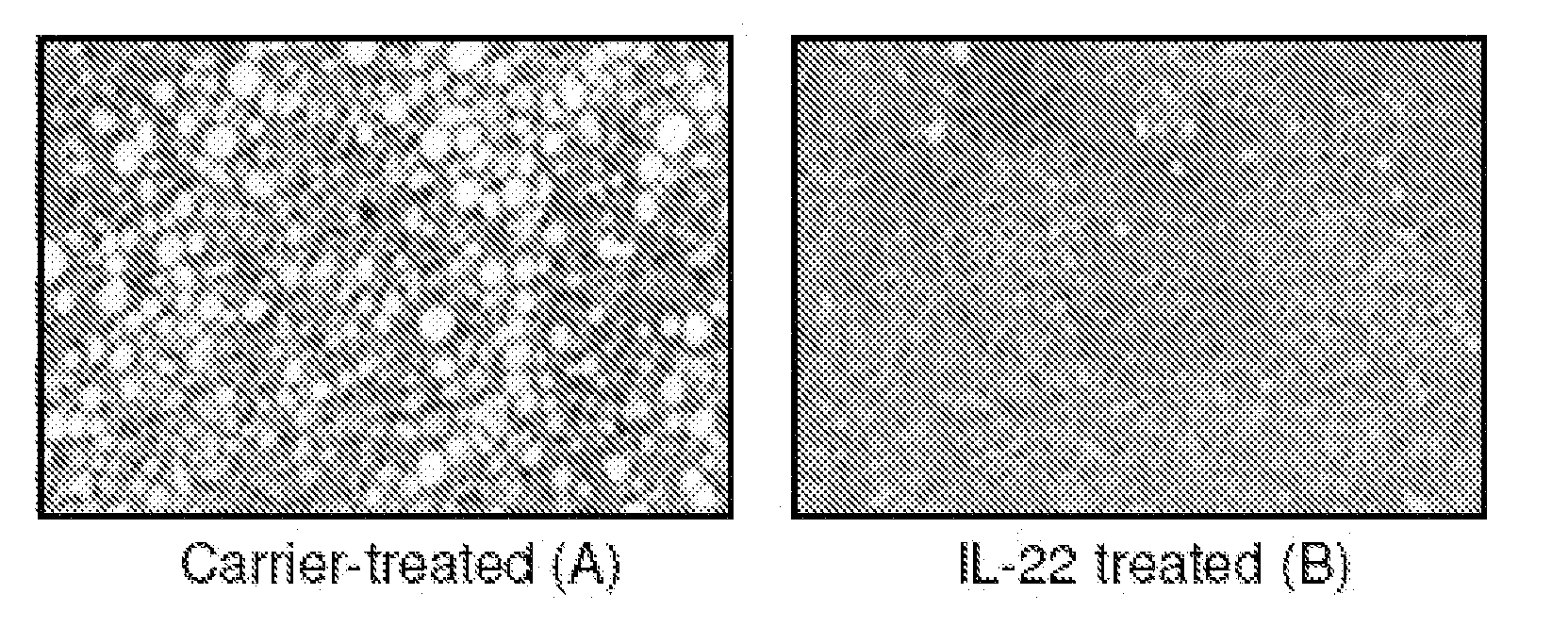
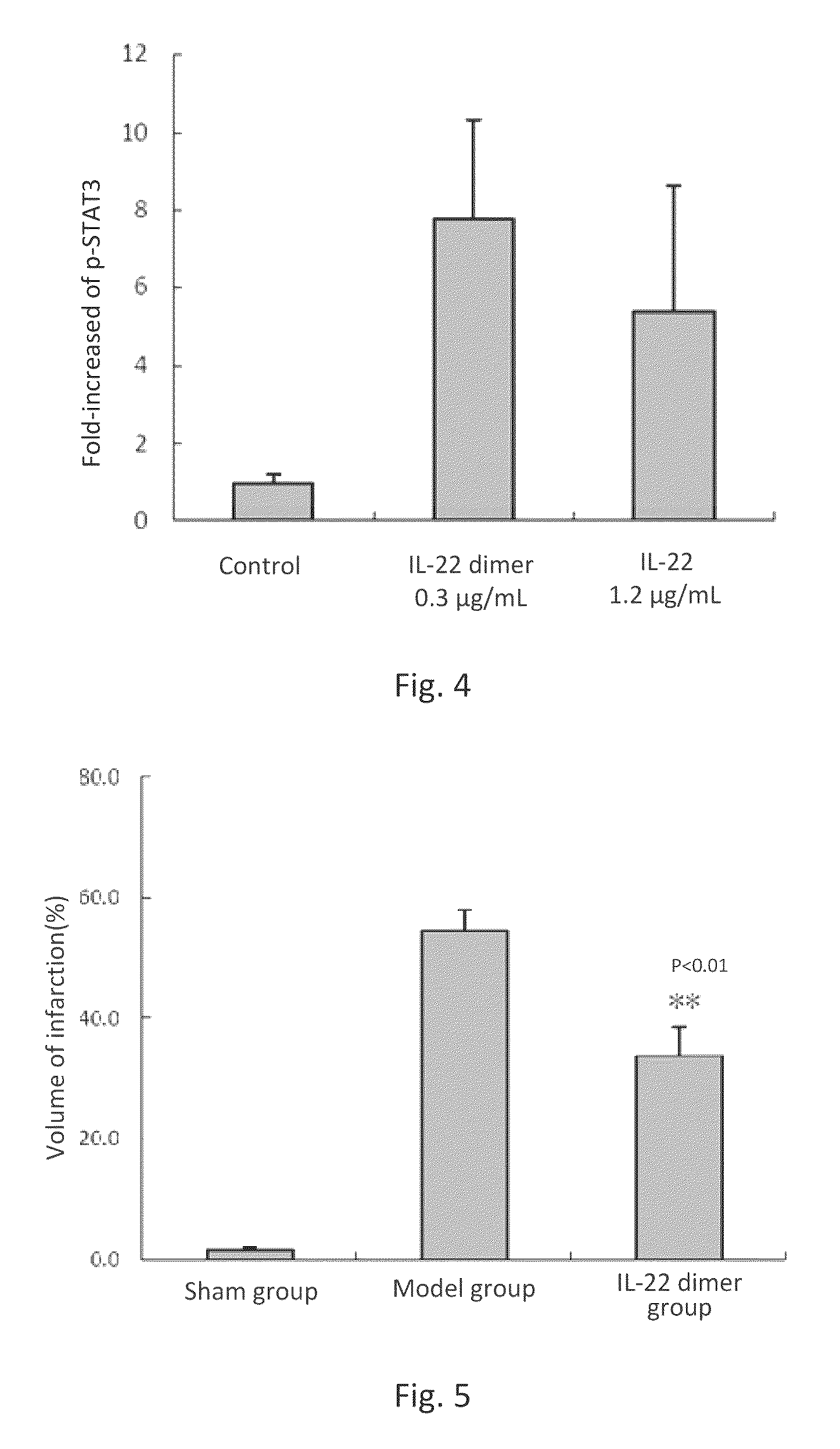
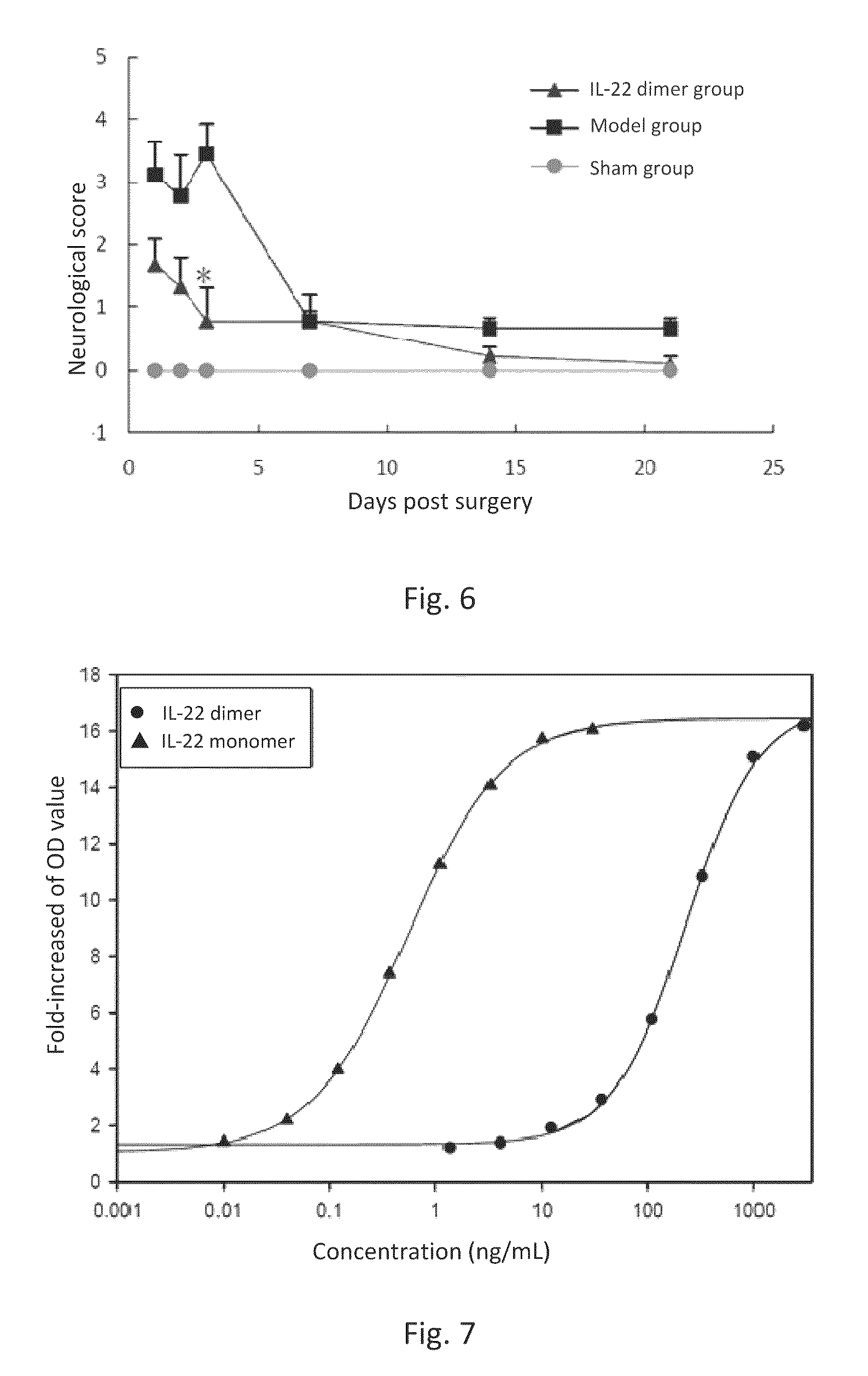
This invention discloses the uses of IL-22 in the treatment and prevention of a nerve damage disease or a neurodegenerative disease. In particular, the invention discloses the uses of IL-22 or IL-22 dimers as follows: (i) can protect neurons to recover the functions of injured neurons after ischemic nerve damage in animals in vivo, thus enabling effective treatment of nerve damage diseases, (ii) can significantly inhibit the loss of dopaminergic neurons in substantia nigra in PD model animal, enhance the functions of dopaminergic neurons, significantly reduce neuronal apoptosis in hippocampus, improve learning and memory capacity of AD model rats, and effectively prevent neuronal loss, thereby enabling more effective treatment of neurodegenerative diseases.
Owner:EVIVE BIOTECHNOLOGY (SHANGHAI) LTD
Use of interleukin-22 in the treatment of fatty liver disease
InactiveUS20110262385A1Lower Level RequirementsReduces Free Fatty AcidPeptide/protein ingredientsMetabolism disorderFatty liverWhite blood cell
The present invention relates to use of interleukin-22 (IL-22) for treating fatty liver disease by decreasing the levels of transaminases. The use of IL-22 in decreasing the levels of transaminases is also provided.
Owner:GENERON (SHANGHAI) CORP LTD
Use of interleukin-22 in treating viral hepatitis
ActiveUS8945528B2Effective treatmentProlong half-life in vivoPeptide/protein ingredientsAntiviralsWhite blood cellViral hepatitis b
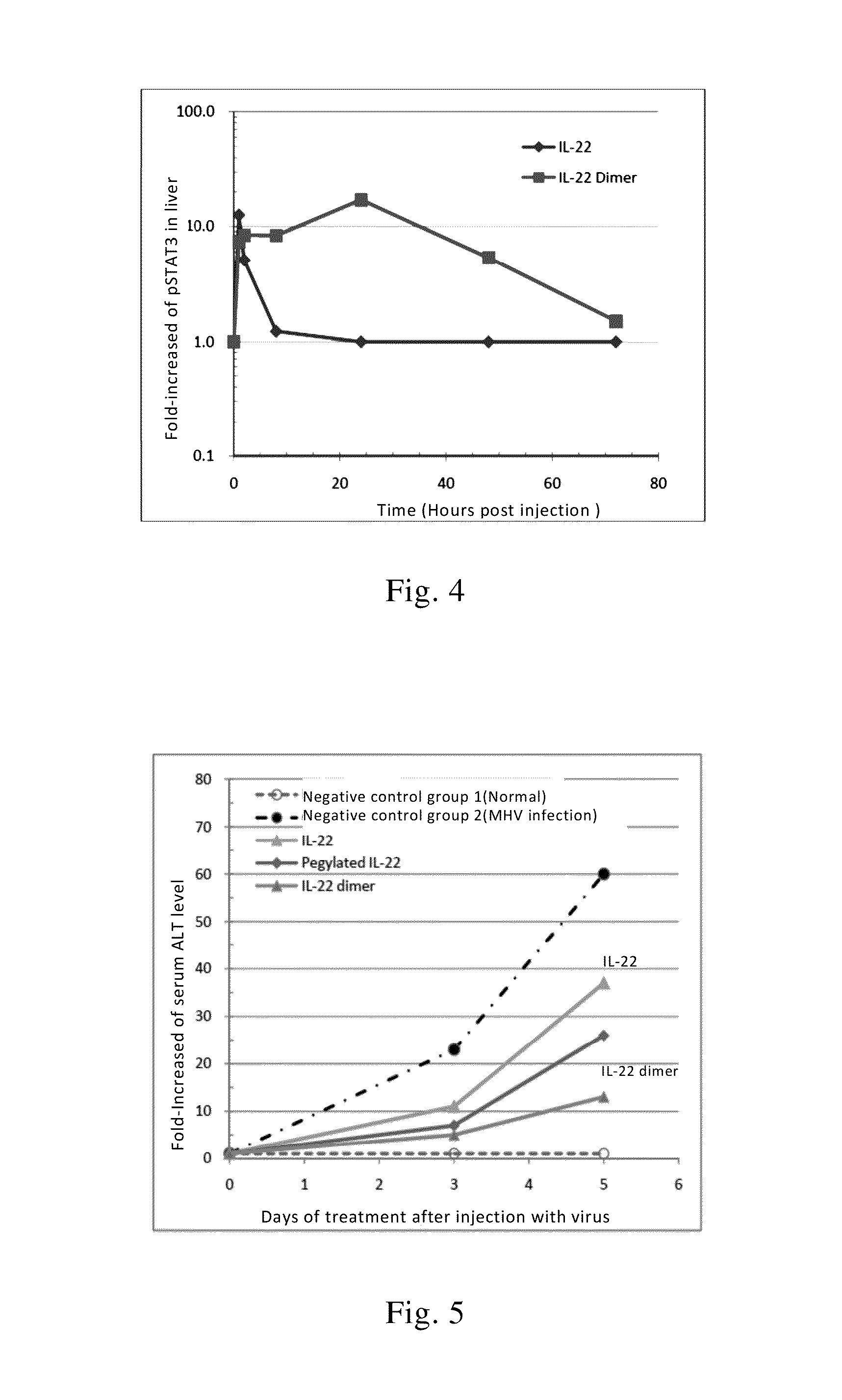
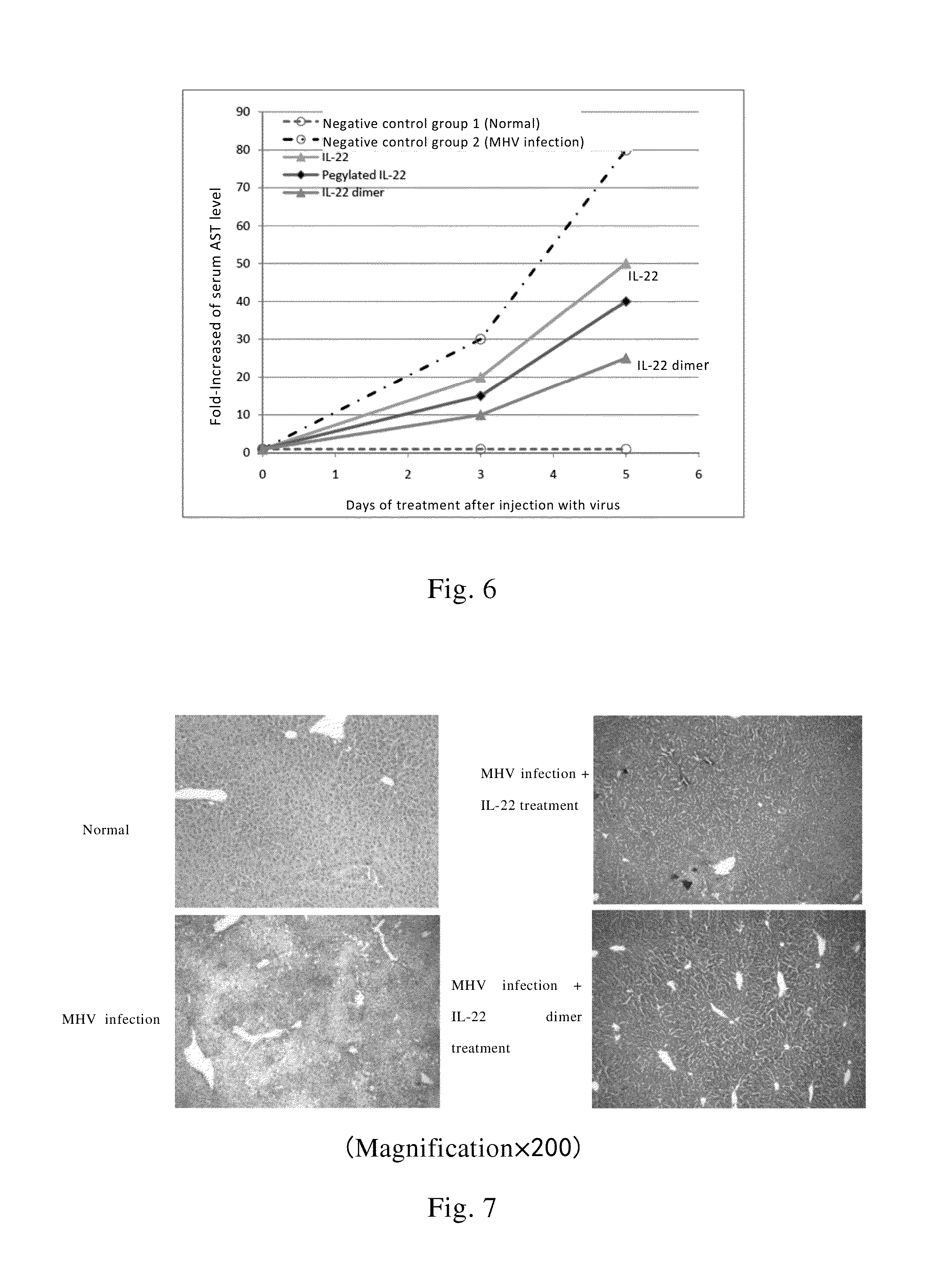
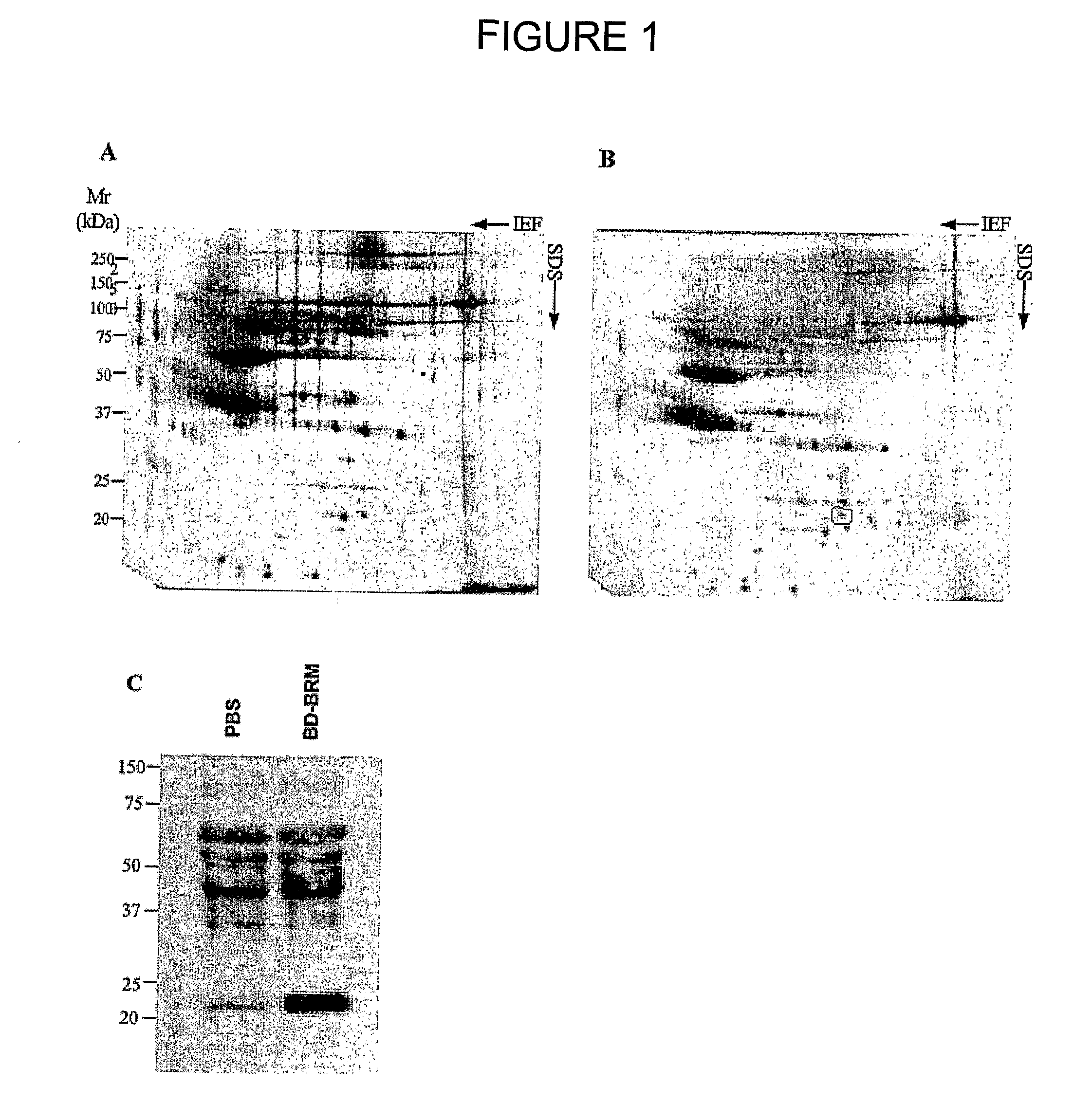
This invention relates to a use of IL-22 in the treatment of viral hepatitis. As illustrated in the examples of this invention, IL-22 can significantly reduce liver damage caused by hepatitis virus, and can significantly reduce the increase of transaminase ALT / AST induced by hepatitis virus. In addition, the IL-22 dimer of this invention can effectively treat viral hepatitis.
Owner:EVIVE BIOTECHNOLOGY (SHANGHAI) LTD
Method for influencing kinase pathways with IL-22
Interleukin-22 interacts with its receptor, referred to as IL-22R, and instigates a series of reactions, leading to activation of various molecules, such as JAK-1, Tyk2, and others. One can identify molecules which mediate this interaction by measuring the activity of one or more of the molecules in the pathway, to identify agonists and antagonists. These, in turn, are useful therapeutic agents, where inappropriate expression of one of the activated molecules is at issue, and requires amelioration.
Owner:WYETH LLC
Use of interleukin 17E for the treatment of cancer
InactiveUS8287851B2Growth inhibitionImprove the level ofOrganic active ingredientsSugar derivativesInterleukin 10Nucleotide
Owner:APTOSE BIOSCIENCES INC
ELISA (Enzyme-linked Immunoassay Assay) plate and kit for predicting ALI (Acute Lung Injury)/ARDS (Acute Respiratory Distress Syndrome) and assessing prognosis of ALI/ARDS
ActiveCN103969439AThe result is accurateEasy to operateDisease diagnosisInterleukin 6Insulin-like growth factor binding
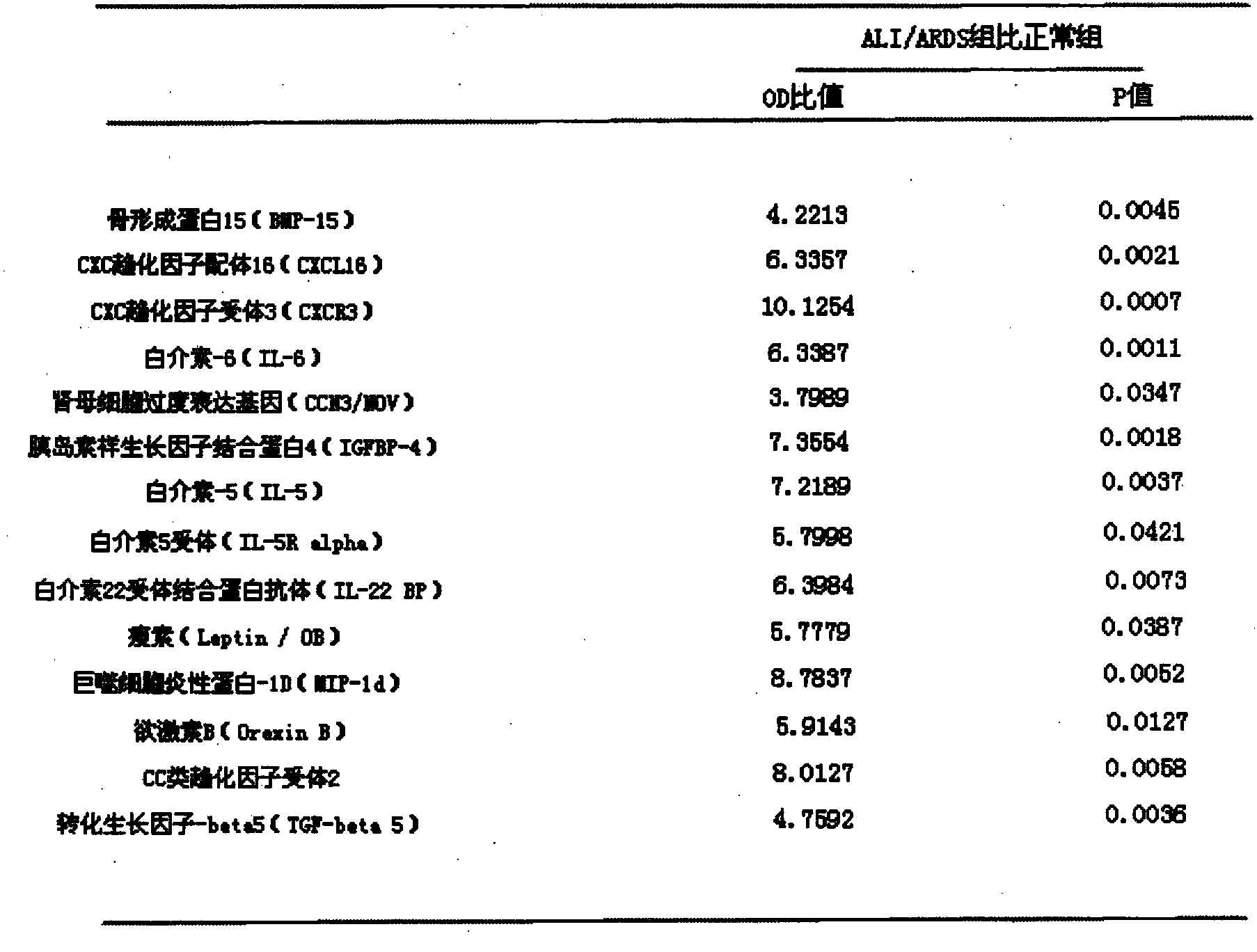
The invention provides an ELISA (Enzyme-linked Immunoassay Assay) plate and kit for predicting ALI (Acute Lung Injury) / ARDS (Acute Respiratory Distress Syndrome) and assessing prognosis of the ALI / ARDS. The ELISA plate comprises a solid phase carrier which is provided with a monoclonal antibody of bone morphogenetic protein 15, a monoclonal antibody of CXC chemokine ligand 16, a monoclonal antibody of CXC chemokine receptor 3, a monoclonal antibody of interleukin-6, a monoclonal antibody of nephroblastoma overexpression genes, a monoclonal antibody of insulin-like growth factor binding protein 4, a monoclonal antibody of interleukin-5, a monoclonal antibody of interleukin 5 receptor, a monoclonal antibody of interleukin 22 receptor binding protein, a monoclonal antibody of leptin, a monoclonal antibody of macrophage inflammatory protein-1D, a monoclonal antibody of orexin B, a monoclonal antibody of CC type chemokine receptor 2, a monoclonal antibody of transforming growth factor-beta5 and blank control holes. The ELISA plate and kit can achieve accurate results and are simple to operate.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL UNIV
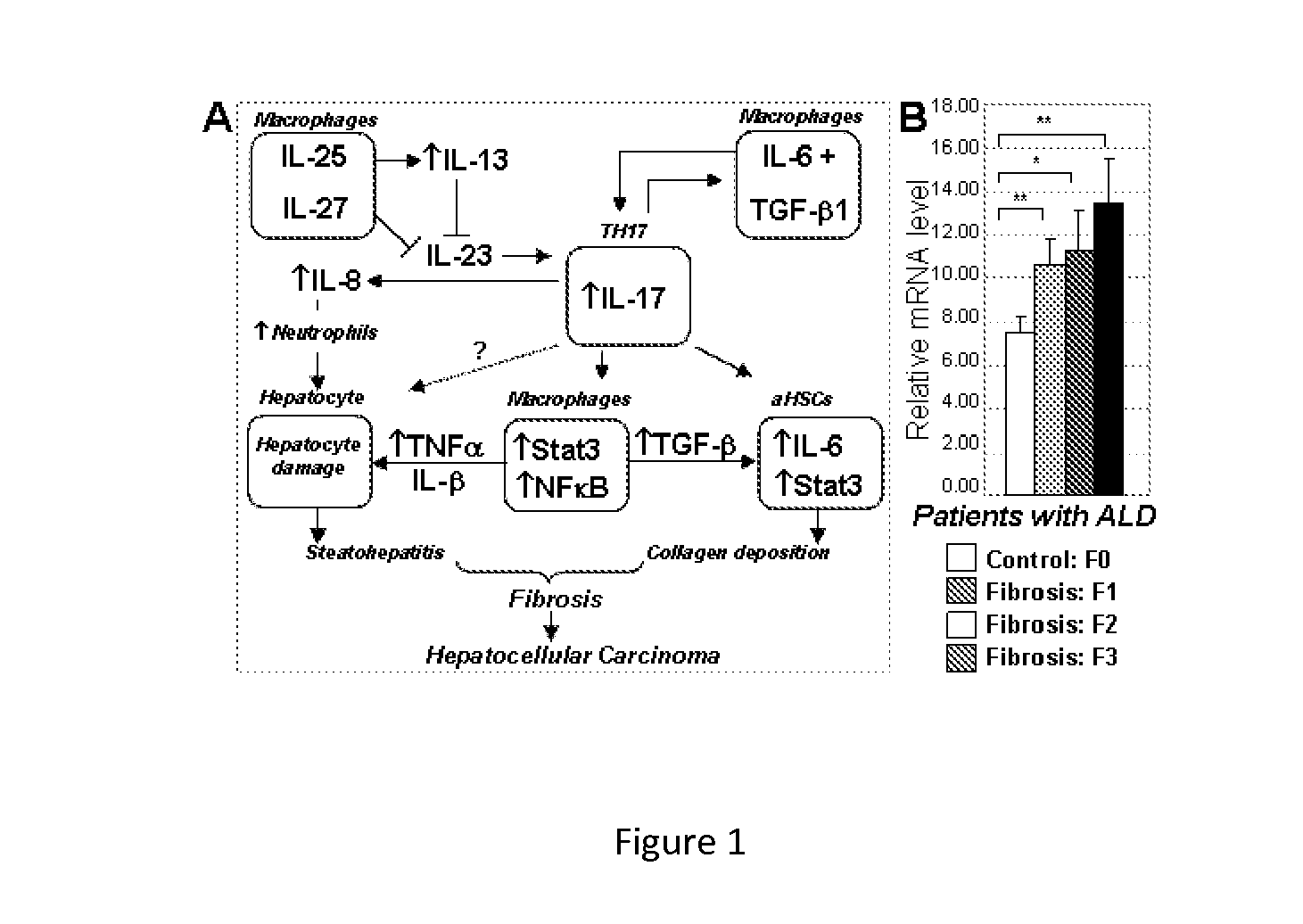
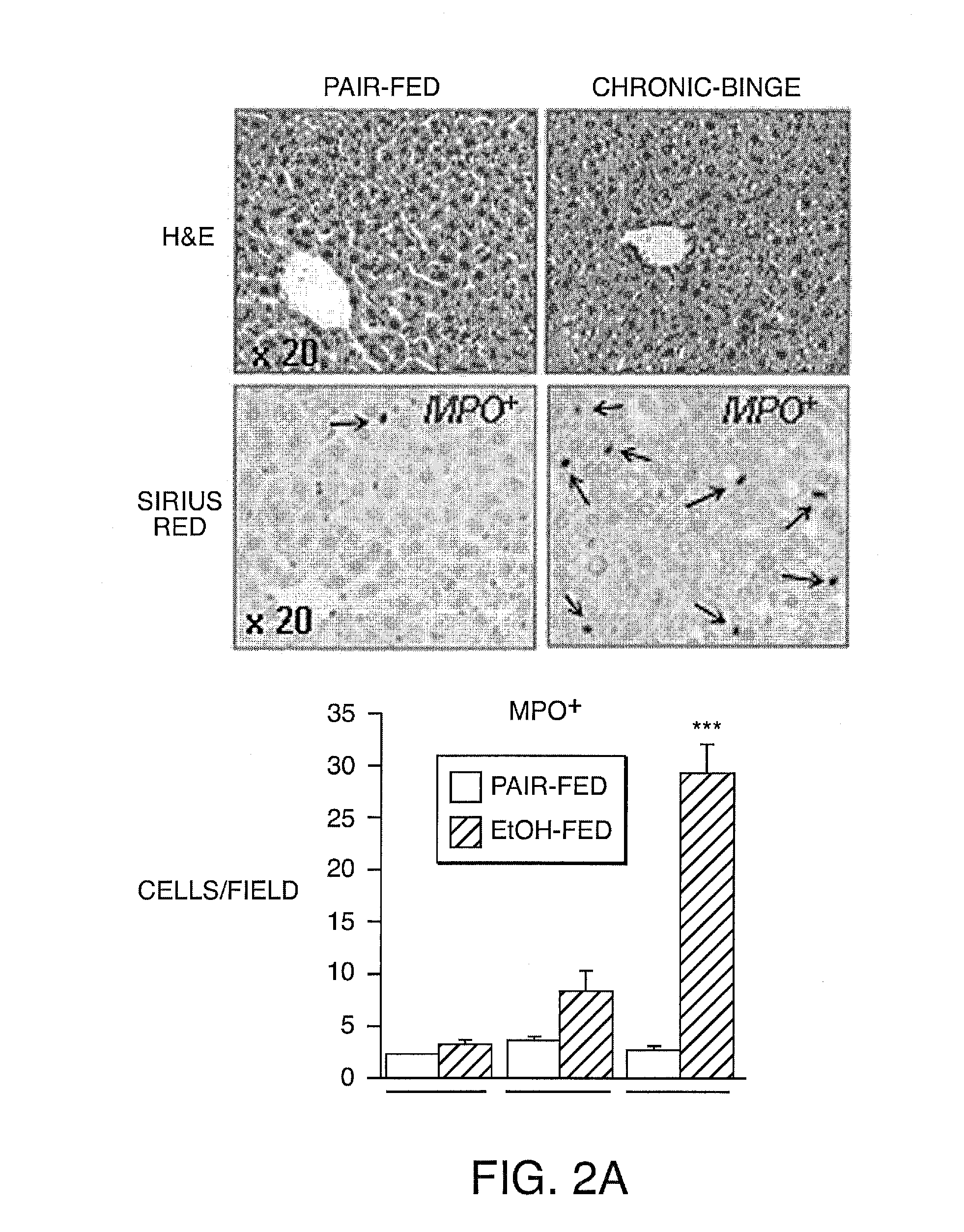
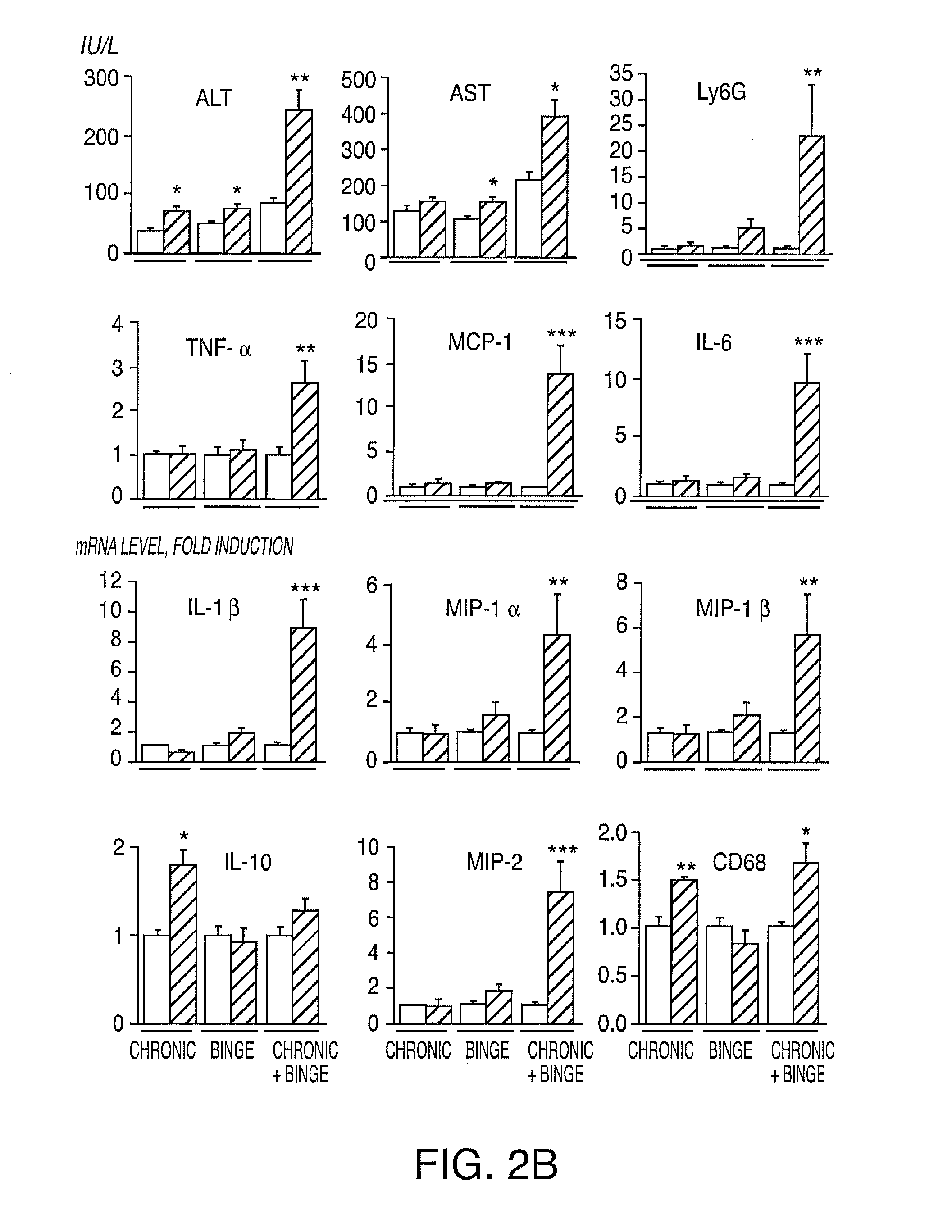
Compositions And Methods For Treating Steatohepatitis, Liver Fibrosis, and Hepatocellular Carcinoma (HCC)
InactiveUS20150004133A1Refine antibody performanceLow affinityPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsHepatocellular carcinomaWhite blood cell
The invention provides methods and compositions for reducing symptoms of steatohepatitis and / or liver fibrosis and / or hepatocellular carcinoma (HCC) in a mammalian subject in need thereof, comprising administering to the mammalian subject a therapeutic amount of a compound that reduces the level of interleukin 17 (IL-17) and / or interleukin 23 (IL-23) and / or signal transducer and activator of transcription 3 (Stat3) and / or Janus kinase 2 (Jak2). The invention's methods may comprise administering to the mammalian subject a therapeutic amount of a compound that increase the level of interleukin 22 (IL-22) and / or interleukin 25 (IL-25) and / or interleukin 27 (IL-27). The invention's methods may comprise administering to the mammalian subject a therapeutic amount of interleukin 22 (IL-22) and / or interleukin 25 (IL-25) and / or interleukin 27 (IL-27).
Owner:RGT UNIV OF CALIFORNIA
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Stanniocalcin-1, Antithrombin-III, Toll-like receptor 2, Triiodothyronine (T3), Thyroxine (T4), Extracellular matrix protein 1, Coagulation factor XIII A chain, Coagulation factor XIII B chain, Interleukin-17F, Interleukin-22, Vitronectin, Progesterone, Estradiol, Growth / differentiation factor 15, and Proprotein convertase subtilisin / kexin type 9 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Uses of interleukin-22(IL-22) in treating and preventing nerve damage diseases or neurodegenerative diseases
ActiveUS9352024B2Reduced infarct volumeInhibiting the loss of dopaminergic neuronsNervous disorderPeptide/protein ingredientsIn vivoDisease cause
This invention discloses the uses of IL-22 in the treatment and prevention of a nerve damage disease or a neurodegenerative disease. In particular, the invention discloses the uses of IL-22 or IL-22 dimers as follows: (i) can protect neurons to recover the functions of injured neurons after ischemic nerve damage in animals in vivo, thus enabling effective treatment of nerve damage diseases, (ii) can significantly inhibit the loss of dopaminergic neurons in substantia nigra in PD model animal, enhance the functions of dopaminergic neurons, significantly reduce neuronal apoptosis in hippocampus, improve learning and memory capacity of AD model rats, and effectively prevent neuronal loss, thereby enabling more effective treatment of neurodegenerative diseases.
Owner:EVIVE BIOTECHNOLOGY (SHANGHAI) LTD
Prevention and/or treatment of multiple organ dysfunction syndrome with interleukin-22
ActiveUS20110268696A1Antibacterial agentsPeptide/protein ingredientsWhite blood cellEmergency medical services
The present invention relates to use an agent for the prevention and / or treatment of multiple organ dysfunction syndrome (MODS) or multiple organ failure (MOF) comprising interleukin-22 (IL-22) as an effective ingredient. The present invention is applicable to prevention of or therapy for diseases from sepsis, septic shock, liver failure, to multiple organ dysfunction syndromes. More particularly, the present invention is useful for an emergency medical service, for treatment of injury caused by a traffic accident, burns, heat attacks, hypercytokinemia or severe infective diseases.
Owner:GENERON (SHANGHAI) CORP LTD
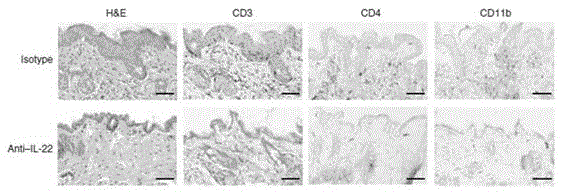
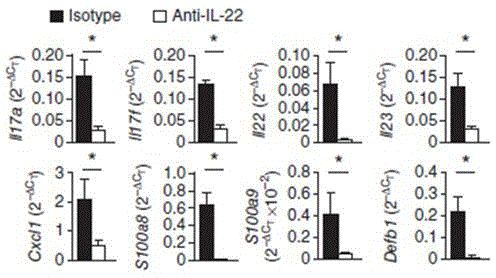
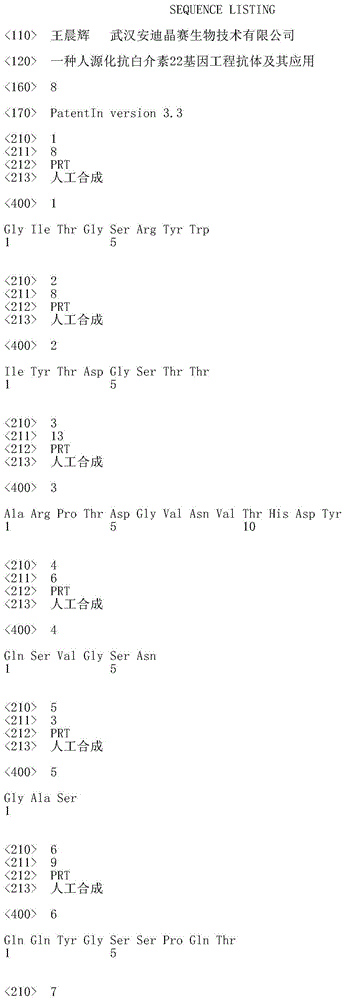
Humanized interleukin-22-resistant genetically engineered antibody and application thereof
The invention relates to the technical field of biology, and particularly discloses a humanized interleukin-22-resistant genetically engineered antibody and a preparation method and application thereof. By means of the genetic engineering technology and the phage display technology, the antibody capable of being combined with human IL-22 can be directly screened out of a human antibody gene pool, and the antibody gene is obtained and expressed. The antibody can be used for treating autoimmune diseases related to Th17 / IL-17 and detecting IL-22.
Owner:武汉原谷生物科技有限责任公司
Prevention and/or treatment of multiple organ dysfunction syndrome with interleukin-22
ActiveUS8956605B2Antibacterial agentsPeptide/protein ingredientsWhite blood cellEmergency medical services
The present invention relates to use an agent for the prevention and / or treatment of multiple organ dysfunction syndrome (MODS) or multiple organ failure (MOF) comprising interleukin-22 (IL-22) as an effective ingredient. The present invention is applicable to prevention of or therapy for diseases from sepsis, septic shock, liver failure, to multiple organ dysfunction syndromes. More particularly, the present invention is useful for an emergency medical service, for treatment of injury caused by a traffic accident, burns, heat attacks, hypercytokinemia or severe infective diseases.
Owner:GENERON (SHANGHAI) CORP LTD
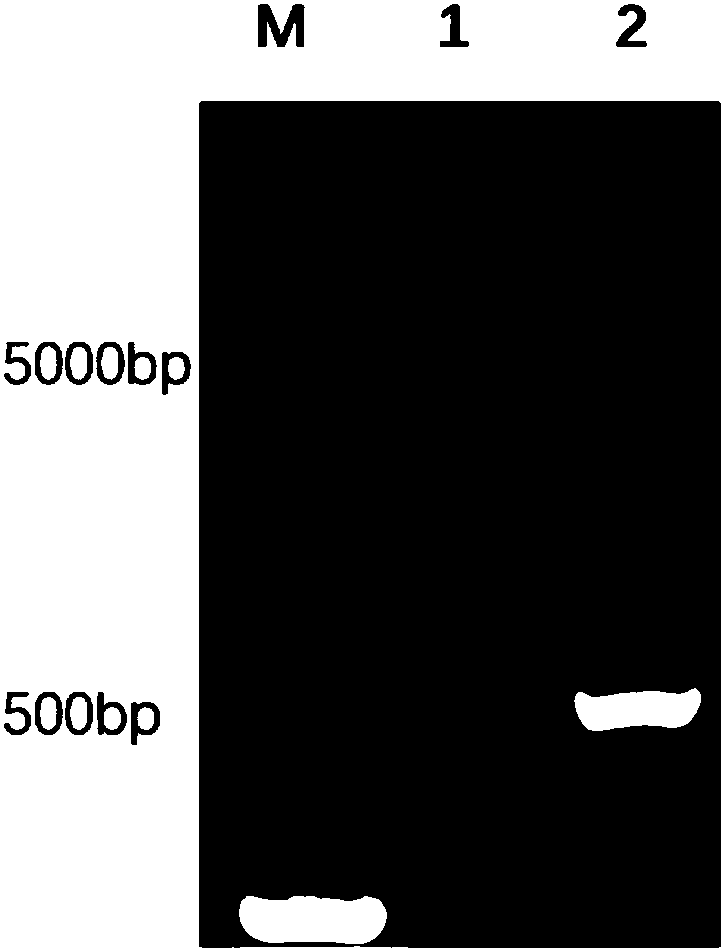
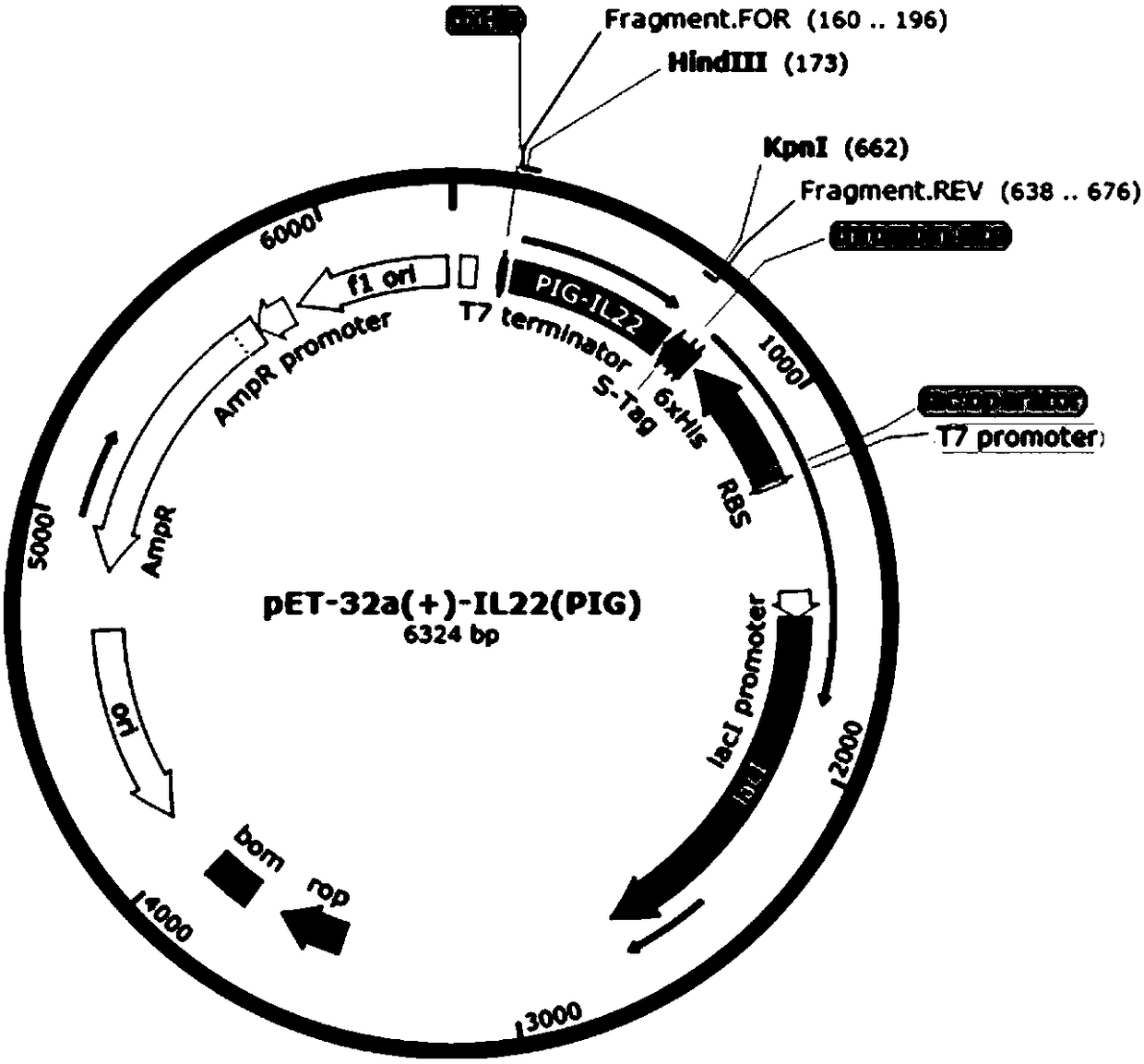
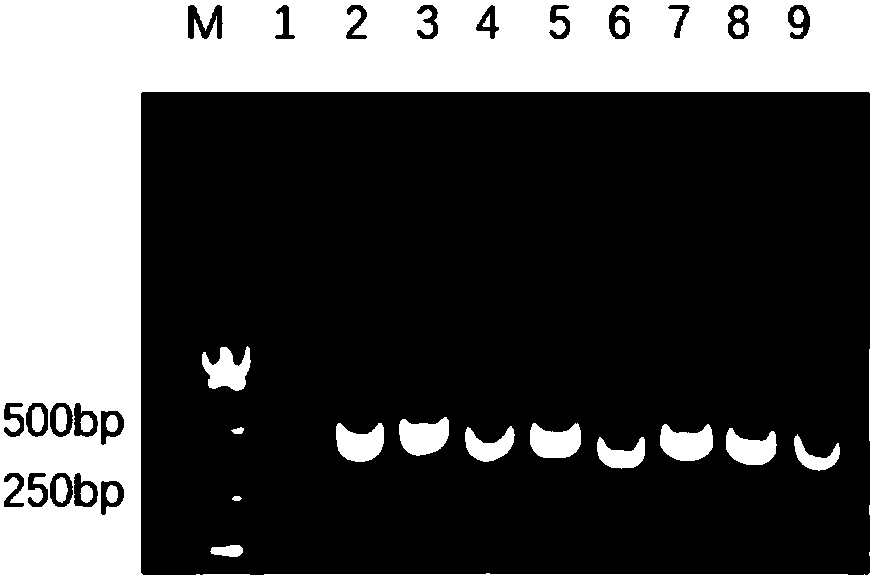
High-efficient expression of recombined swine IL 22 in escherichia coli and its application
ActiveCN108070599AProcess stabilityFree from harmAntibacterial agentsAntimycoticsBiotechnologyPurification methods
The invention discloses a gene for encoding recombined swine IL 22. The invention further discloses a recombined expression carrier containing a recombined gene, a transgene cell system, a transgene recombined bacterial and a host cell. The invention further discloses a recombined swine IL 22 and its extraction and purification method. The invention further discloses an application of recombined plasmid pET-32a(+)-PIL-22, recombined strain and recombined protein in anti-apoptosis and prevention of escherichia coli infection. The carrier pET-32a(+) of the expression recombined swine IL 22 structured in the invention contains His label, the recombined swine IL 22 protein extracted from escherichia coli is connected with the His label; through a His pillar, the highly purified recombined swine IL 22 protein is acquired; the expression output of the recombined swine IL 22 protein is counted through Quantity One software, the purity is up to 95.7%.
Owner:NANJING AGRICULTURAL UNIVERSITY
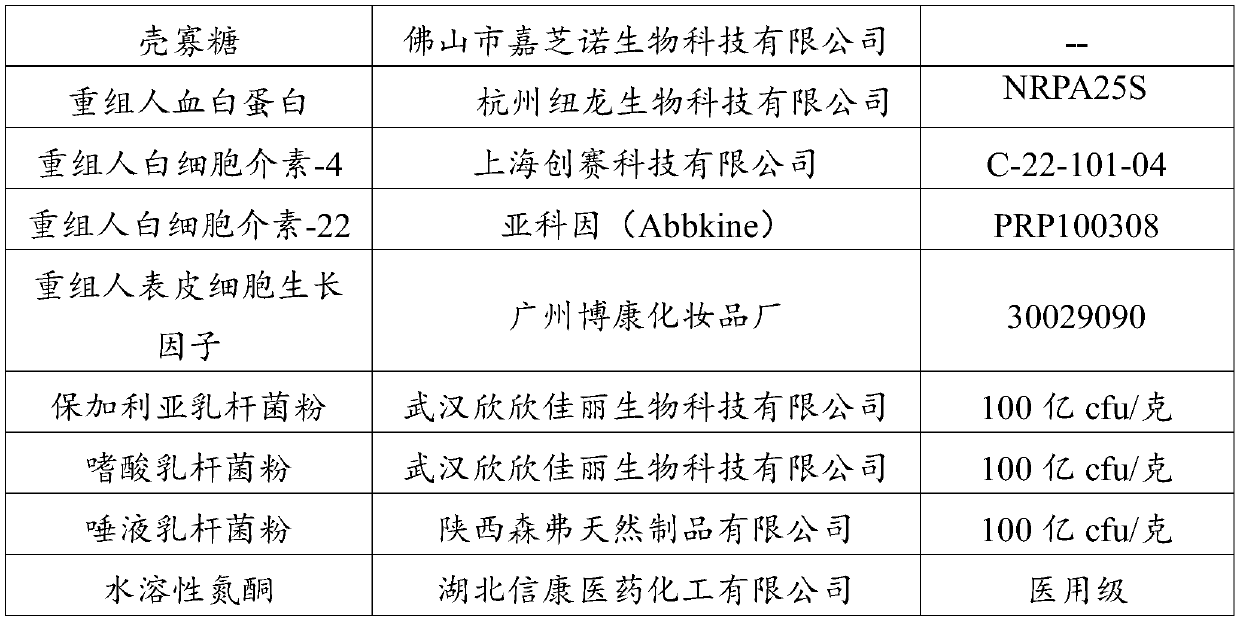
Vagina injection agent for treating uterine prolapse and preparation method of vagina injection agent
PendingCN110522716AHigh purityQuality improvementPeptide/protein ingredientsPharmaceutical delivery mechanismDamages tissueTissue repair
The invention discloses a vagina injection agent for treating uterine prolapse. The vagina injection agent is prepared from the following components in parts by weight: 5-20 parts of beta-glucan, 1-10parts of lycium barbarum polysaccharide, 1-10 parts of chitosan oligosaccharide, 5-15 parts of recombinant human serum albumin, 0.05-0.5 part of recombinant human interleukin-22, 0.1-0.6 part of recombinant human interleukin-4, 0.3-1.5 parts of a recombinant human epidermal growth factor, 10-15 parts of active lactobacillus powder, 0.1-1 part of a penetration enhancer, 1-5 parts of a bio-adhesiveagent, 40-60 parts of a buffer solution and 50-100 parts of normal saline. The vagina injection agent directly acts on lesion parts, local drug concentration is high, the vagina micro-environment canbe effectively improved, the micro-ecological balance in a vagina and the normal micro-environment of a cervix are maintained, and local and body immune functions are improved; and the vagina injection agent can further promote cell regeneration, promote collagen synthesis, improve skin elasticity, accelerate repair of damaged tissue, promote tension recovery of pelvic floor muscles, fascia and uterus ligament, and can effectively improve uterine prolapse; and recurring is not prone to appearing after cure, using is convenient and safe, and the effect is fast.
Owner:广东圆康再生医学科技开发有限公司
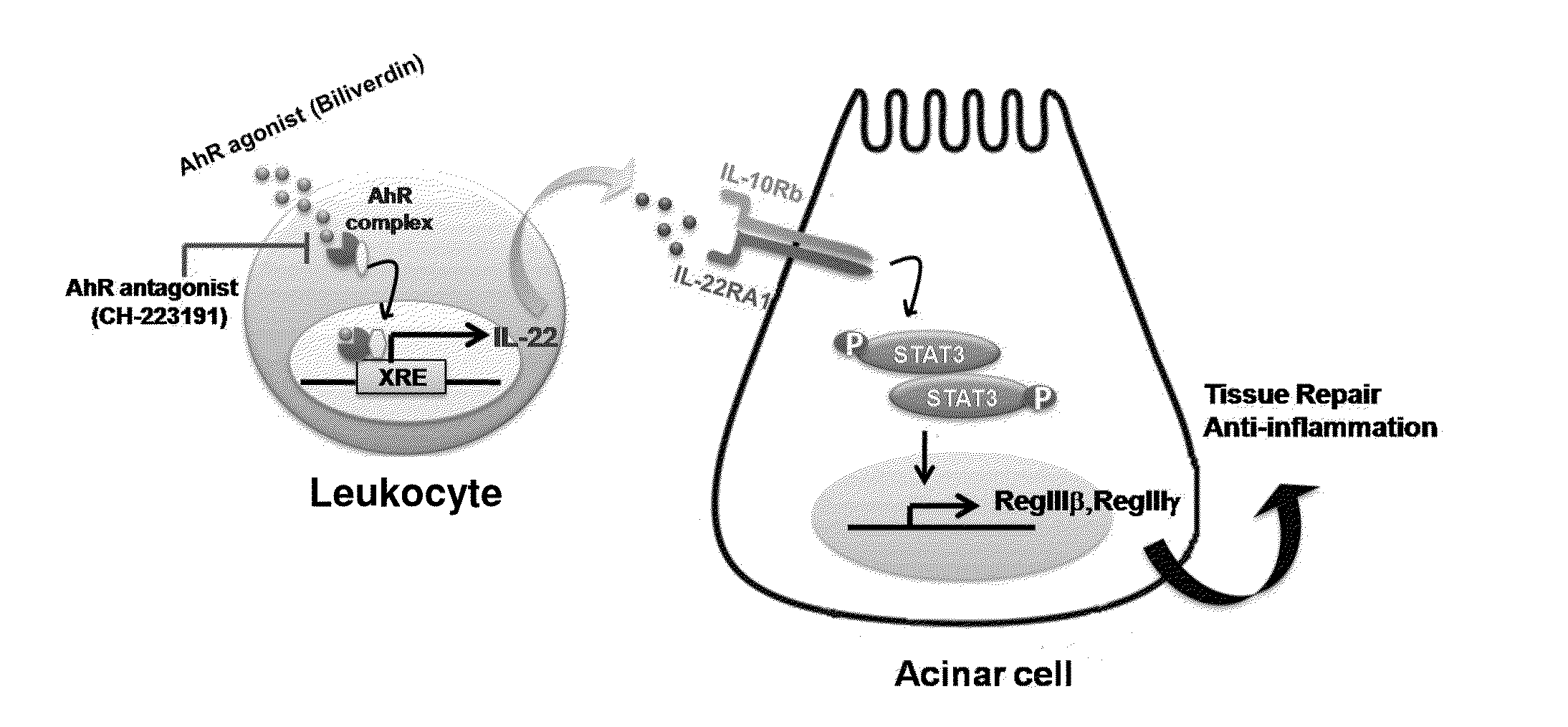
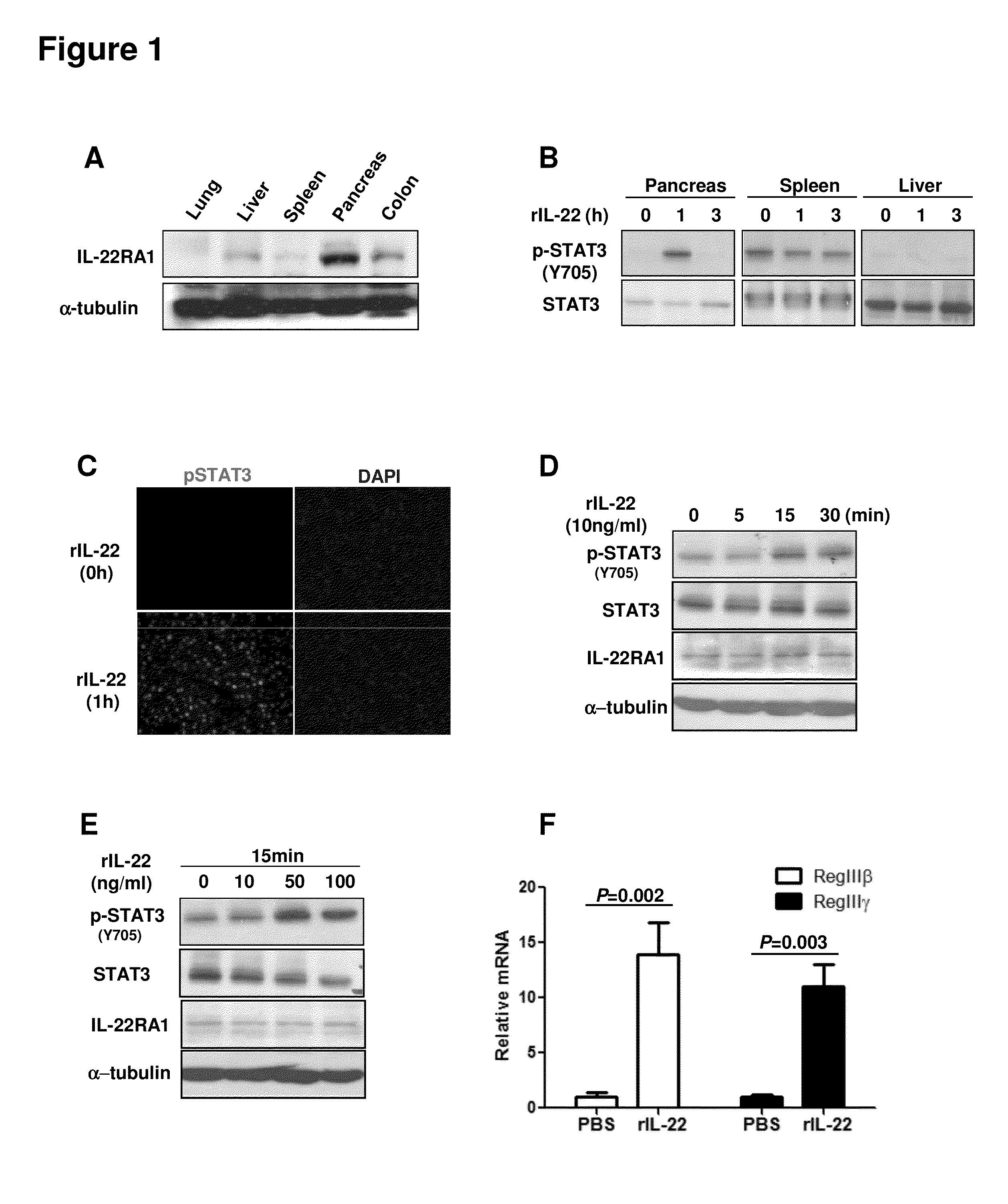
Methods to treat pancreatic inflammation and associated lung injury through regulation of pancreatic interleukin-22 expression
Methods for use of a composition comprising agents that increase pancreatic interleukin-22 production in the treatment of pancreatic inflammatory disorders including pancreatitis-associated acute lung injury.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Medicine use of interleukin-22
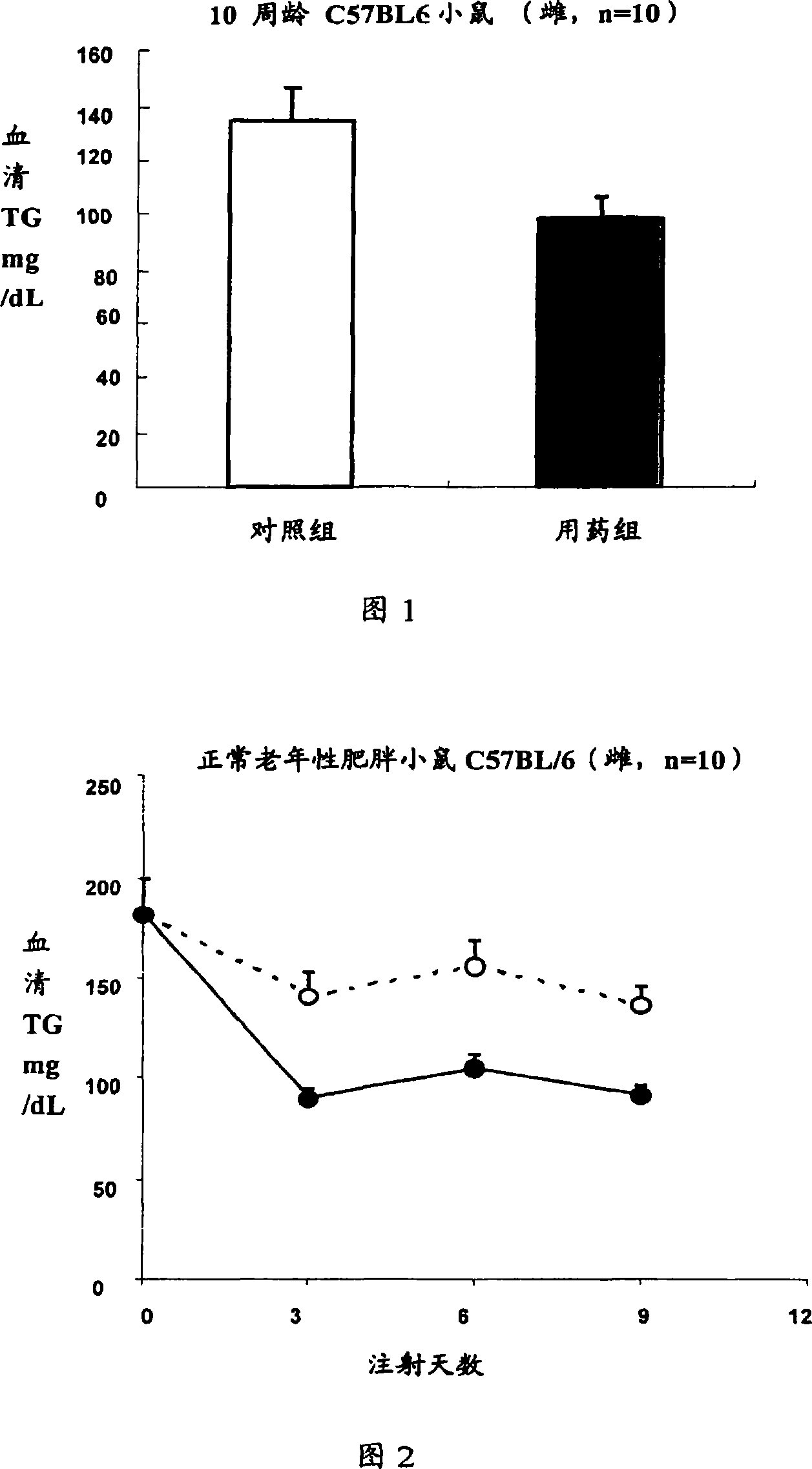
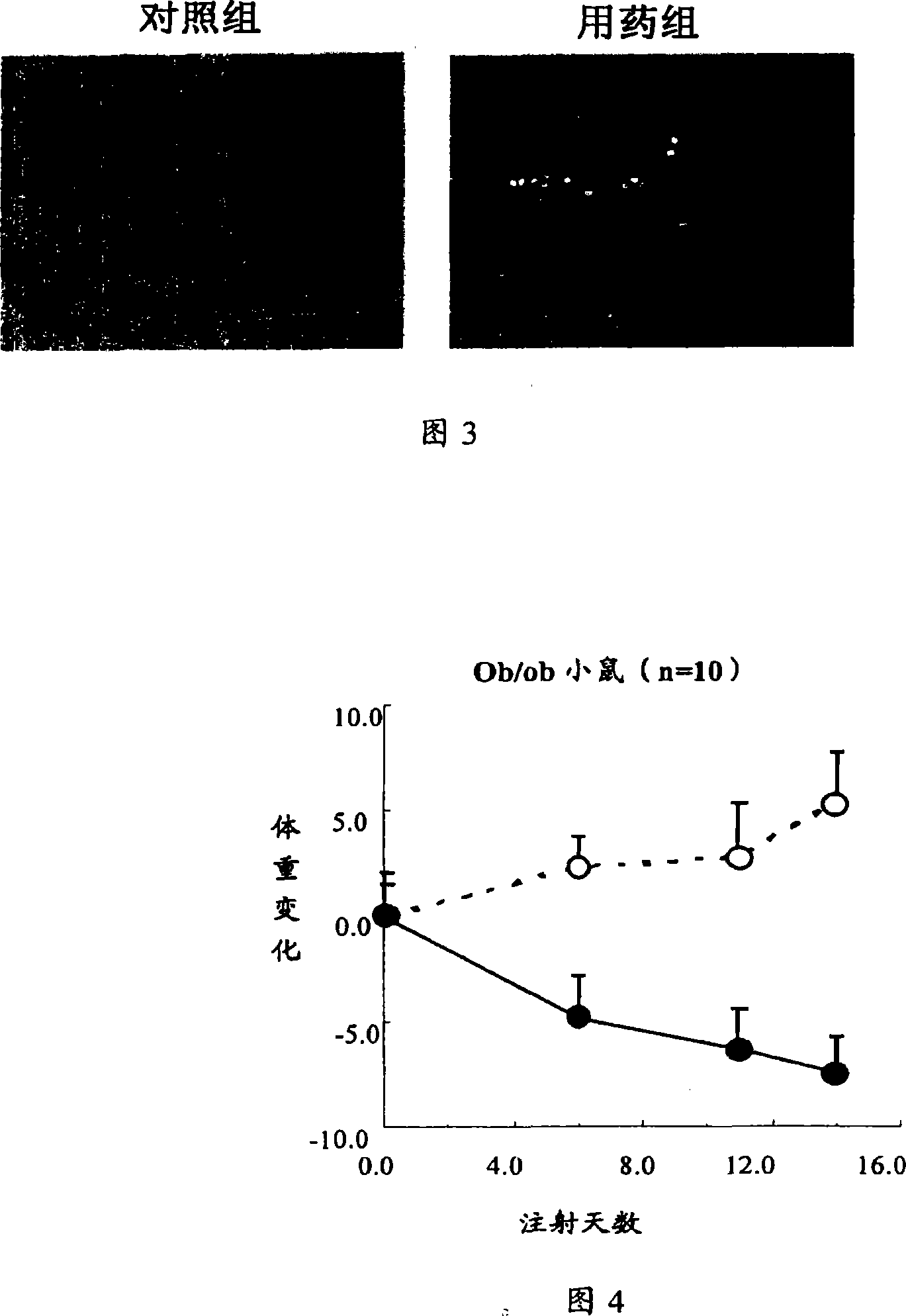
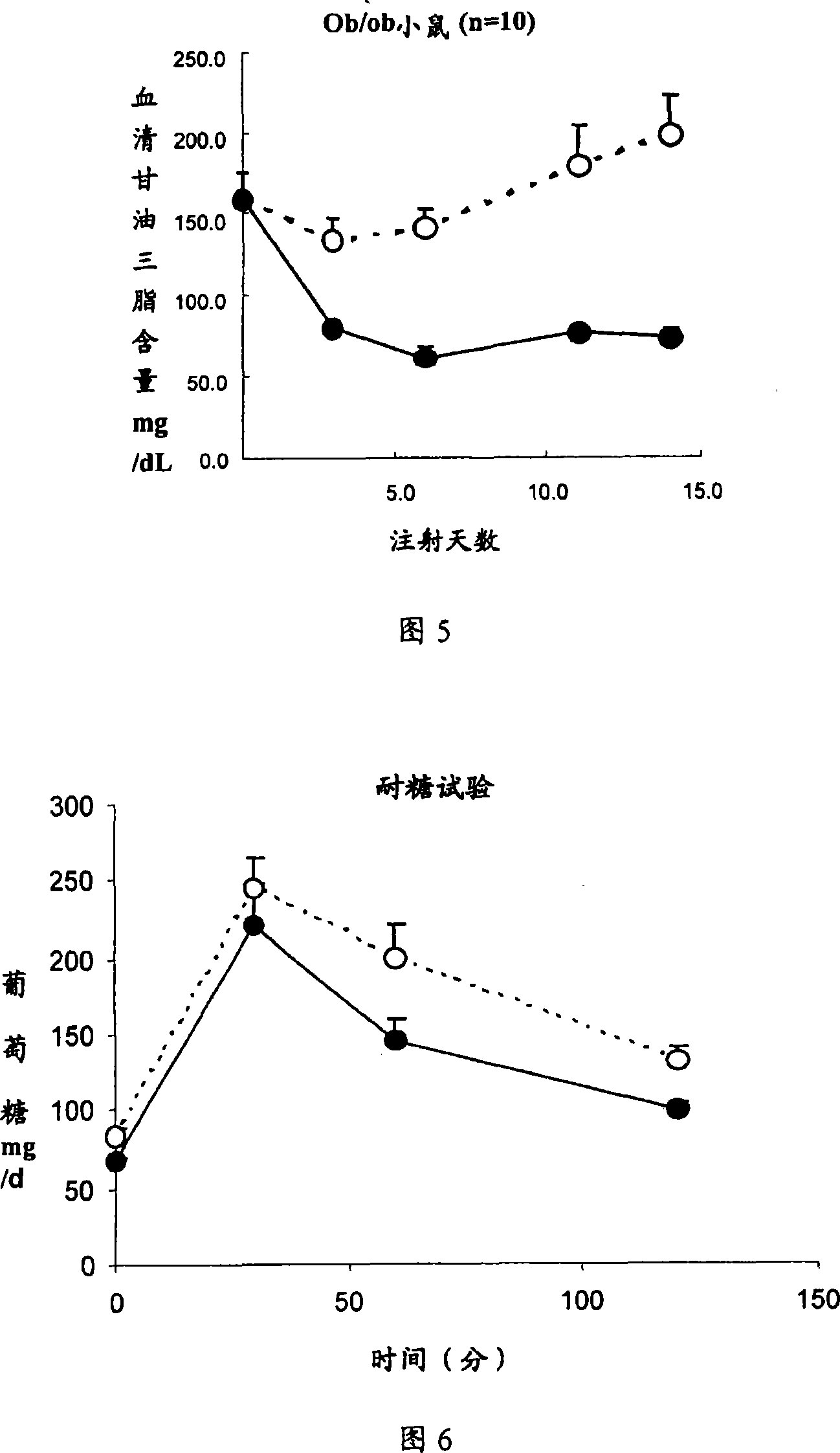
ActiveCN101219208APeptide/protein ingredientsMetabolism disorderHypertriglyceridemiaSecondary hyperlipidemia
The invention relates to the medical application of Interleukin-22, in particular to the application of Interleukin-22 for treating hyperlipidemia, hypertriglyceridemia and obesity and (or) diabetes. The invention also relates to the use of preparing medicines for treating hyperlipidemia, hypertriglyceridemia and obesity and (or) diabetes. The Interleukin-22 of the invention is human Interleukin-22, recombination human Interleukin-22, rat Interleukin-22 and (or) recombination rat Interleukin-22.
Owner:亿一生物制药(北京)有限公司
Il22 immunoconjugates
InactiveUS20170305989A1High activityNervous disorderPeptide/protein ingredientsAutoimmune thyroid diseaseAutoimmune disease
The application relates to a conjugate comprising interleukin-22 (IL22) and an antibody molecule. The antibody molecule preferably binds an antigen associated with angiogenesis, such as the ED-A isoform of fibronectin. In particular, the application relates to the therapeutic use of such conjugates in the treatment of a disease / disorder, such as autoimmune diseases, including inflammatory bowel disease (IBD).
Owner:PHILOGEN SRL LLC
Use of interleukin 17e for the treatment of cancer
InactiveUS20110158936A1Growth inhibitionImprove the level ofOrganic active ingredientsSugar derivativesInterleukin 10Interleukin 5
The use of interleukin 17E to inhibit tumour growth in a subject is provided. The interleukin 17E can be provided to the subject exogenously, as an interleukin 17E polypeptide or a polynucleotide encoding an interleukin 17E polypeptide, or it can be provided by stimulating production of endogenous interleukin 17E. Also provided is the use of interleukin 17E in combination with one or more anti-cancer therapeutics for inhibiting tumour growth in a subject. Anti-cancer therapeutics include, for example, standard chemotherapeutic drugs, immunotherapeutics, radiation, gene therapy, hormone manipulation and antisense therapy.
Owner:APTOSE BIOSCIENCES INC
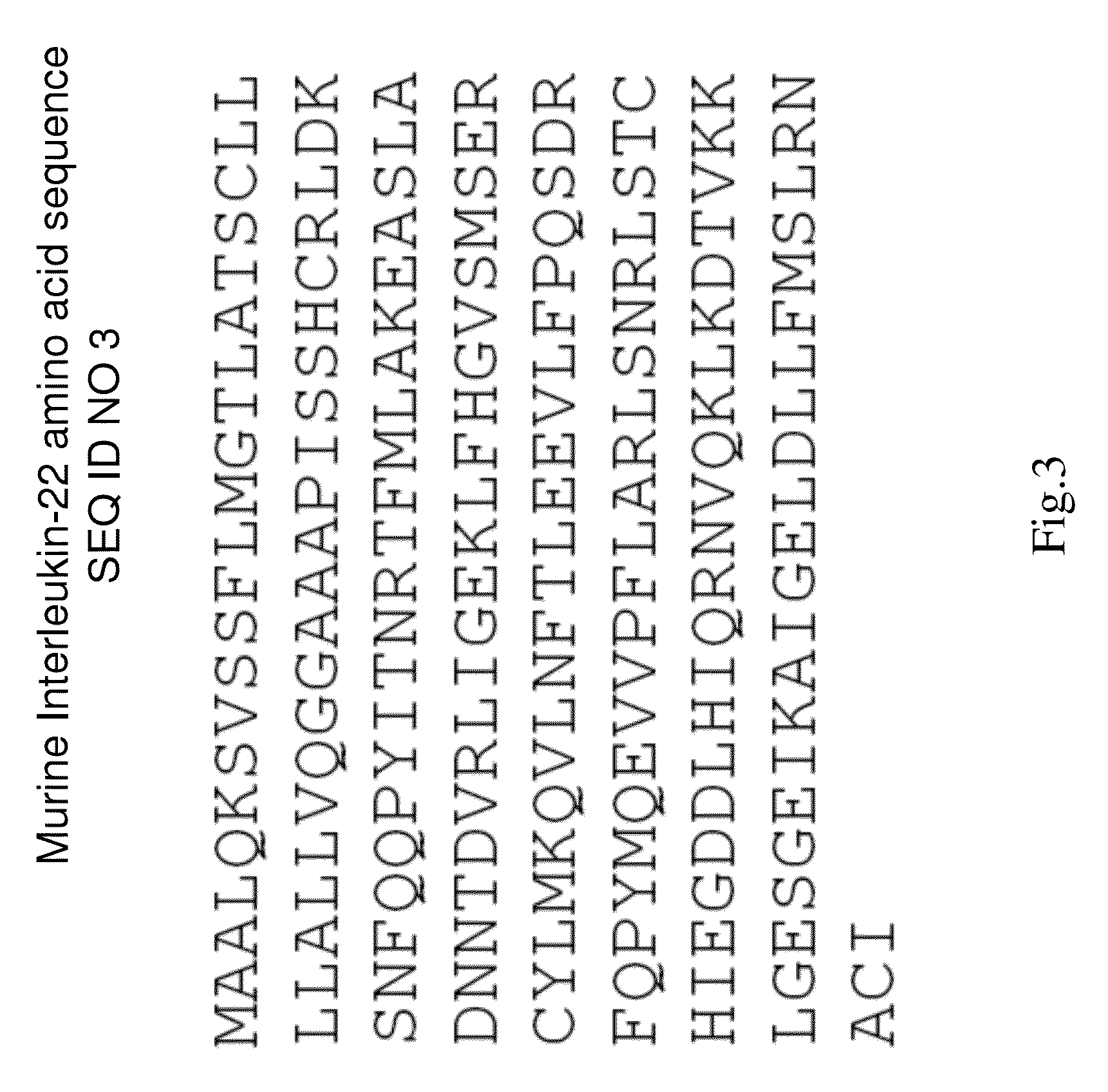
Application of recombinant porcine interleukin 22 in preparation of drugs for improving production performance of pigs
PendingCN111346218AGood effectIncreased average daily feed intakePeptide/protein ingredientsSexual disorderBiotechnologyPharmaceutical drug
The invention provides application of IL22 in the preparation of drugs for improving the production performance of pigs. A DNA sequence of a recombinant porcine interleukin 22 is shown as SEQ ID NO.3.The drugs can be a fermentation broth obtained by eukaryotic fermentation of IL22, or an oral preparation prepared by IL22 combined with a pharmaceutically acceptable auxiliary material or carrier. The food intake of pigs can be significantly increased, the diarrhea rate of pigs can be reduced, so that the feed conversion rate is reduced, and the growth performance of pigs is improved.
Owner:四川华德生物工程有限公司
Application of immune cytokine interleukin-22 in preparation ofanti-depression drug
ActiveCN112656936AHas antidepressant propertiesQuick effectNervous disorderPeptide/protein ingredientsSide effectPharmaceutical drug
The invention belongs to the technical field ofanti-depression drugs, and relates to application of immune cell factor interleukin 22 in preparation ofanti-depression drugs. The anti-depression drug containing the immune cell factor interleukin 22 or the composition thereof has the anti-depression characteristic, is quick in onset time, exact and remarkable in treatment effect and small in side effect, and the immune cell factor interleukin 22 has great application value in research and development of the anti-depression drug and treatment of depression.
Owner:PEKING UNIV +1
Preparation and application of porcine interleukin 17 and 22 co-expression antibiotic-replacing biological preparation
ActiveCN113004424APromote secretionEnhance humoral immunityPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunocompetenceWhite blood cell
The invention discloses preparation and application of a porcine interleukin 17 and 22 co-expression antibiotic-replacing biological preparation. The invention provides a fusion protein. The fusion protein comprises porcine interleukin 17 and porcine interleukin 22. The protein is specifically a protein shown in a sequence 1 or a sequence 3. Nucleic acid molecules encoding the protein also belong to the protection range of the invention. Expression cassettes, recombinant vectors, transfected cells or recombinant microorganisms with the nucleic acid molecules all belong to the protection range of the invention. The invention also provides a culture product of the transfected cell or a fermentation product of the recombinant microorganism. The invention also protects the application of the biological material: preparation of a product for improving animal immunocompetence; improving the animal immunity; preparing a vaccine; resisting pathogenic microorganism infection; the biological material is the protein or the nucleic acid molecule or the expression cassette or the recombinant vector or the transfected cell or the recombinant microorganism or the culture product or the fermentation product.
Owner:四川三优康生物技术有限公司
Methods and pharmaceutical compositions for the prophylactic treatment of bacterial superinfections post-influenza
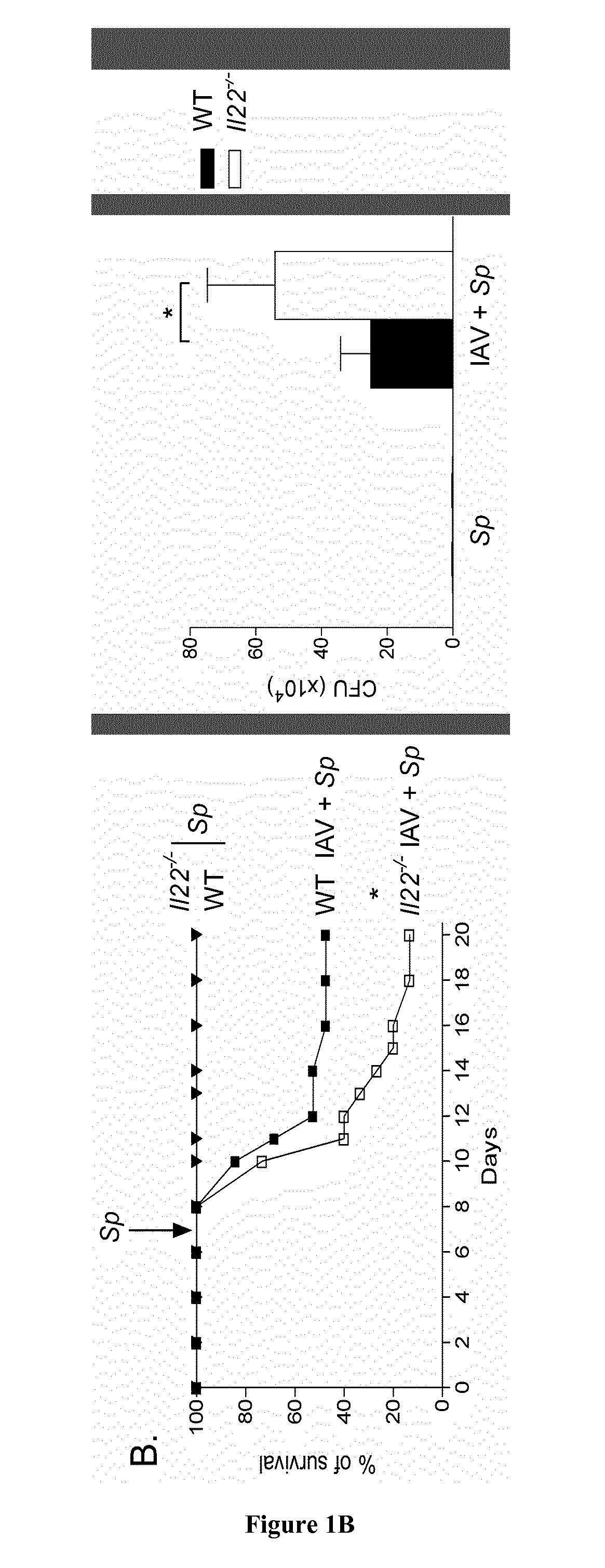
InactiveUS20150246095A1Good curative effectLow toxicityPeptide/protein ingredientsMicrobiological testing/measurementProphylactic treatmentInterleukin 22
The present invention relates to methods and pharmaceutical compositions for the prophylactic treatment of bacterial superinfections post-influenza. In particular, the present invention relates to an interleukin 22 (IL-22) polypeptide for use in the prophylactic treatment of bacterial superinfections post-influenza in a subject in need thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
A method of inhibiting the expression of il-22 in activated t-cells
The present invention relates to a method of inhibiting the upregulation of the expression of the proinflammatory cytokine interleukin-22 (IL-22) in activated and differentiated human T-cells, said upregulation being induced by administration of a therapeutically effective amount of a phosphodiesterase 4 (PDE4) inhibitor. The method comprises administering, either sequentially to or simultaneously with the administration of the PDE4 inhibitor, a vitamin D receptor agonist in an amount 3) sufficient to inhibit the upregulation of IL-22 expression.
Owner:LEO PHARMA AS
Interleukin-22 polypeptides, nucleic acids encoding the same and methods for the treatment of pancreatic disorders
The present invention is directed to interleukin-22 polypeptides and nucleic acid molecules encoding those polypeptides. Also provided herein are vectors and host cells comprising those nucleic acid sequences, chimeric polypeptide molecules comprising the polypeptides of the present invention fused to heterologous polypeptide sequences, antibodies which bind to the polypeptides of the present invention and to methods for producing the polypeptides of the present invention.
Owner:GENENTECH INC
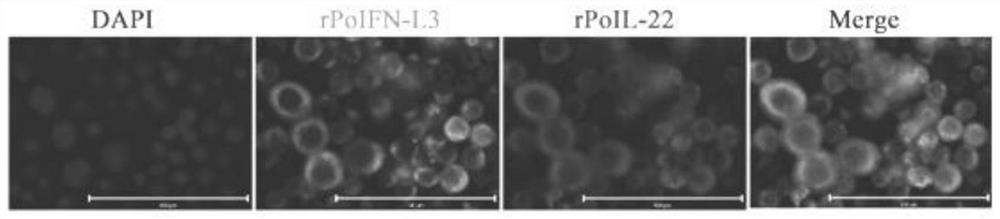
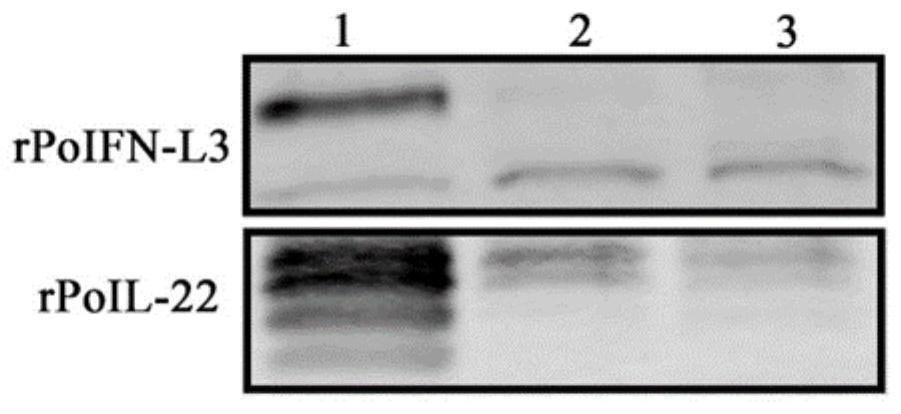
Recombinant baculovirus co-expressing porcine interferon L3 and porcine interleukin 22 and application
PendingCN114736878APeptide/protein ingredientsMicroorganism based processesDrug biological activitySynexpression
The invention discloses recombinant baculovirus for co-expression of porcine interferon L3 and porcine interleukin 22 by using a baculovirus / insect cell expression system. Indirect immunofluorescence and Western-blot prove that the rPoIFN-L3 and the rPoIL-22, which are co-expressed, can be secreted and expressed in a cell supernatant. The co-expressed rPoIFN-L3 and rPoIL-22 have good biological activity, and can be used for effectively inhibiting the infection of TGEV / PEDV / PDCoV in vitro respectively. In addition, the research finds that the rPoIFN-L3 and the rPoIL-22 have a synergistic anti-TGEV / PEDV effect in vitro when the concentration is 50ng / mL. A large number of rPoIFN-L3 and rPoIL-22 with biological activity can be obtained through the protein expression system, and a foundation is laid for further realization of industrialized preparations for resisting porcine intestinal coronavirus infection.
Owner:秦皇岛摩登狗生物科技有限公司 +3
A kind of humanized anti-interleukin 22 genetic engineering antibody and its application
The invention relates to the technical field of biology, and particularly discloses a humanized interleukin-22-resistant genetically engineered antibody and a preparation method and application thereof. By means of the genetic engineering technology and the phage display technology, the antibody capable of being combined with human IL-22 can be directly screened out of a human antibody gene pool, and the antibody gene is obtained and expressed. The antibody can be used for treating autoimmune diseases related to Th17 / IL-17 and detecting IL-22.
Owner:武汉原谷生物科技有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com