Human interleukins-22 mutant as well as construction method and use thereof
An interleukin, mutant technology, applied in the direction of interleukin, cytokine/lymphokine/interferon, application, etc., can solve problems such as inability to accurately infer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Fishing of wild-type hIL22 gene and construction of recombinant expression vector
[0084] 1.1 Extraction of total RNA
[0085] Dilute 1ml of fresh peripheral blood by 2 times with PBS containing 2mmol / L EDTA, spread it on an equal volume of lymphocyte separation medium (purchased from Shanghai Huajing Biological Company), centrifuge at 800r / min to make layers, and then use capillary Aspirate the buffy coat cells. After washing with Hanks solution for 3 times, the cells were suspended in 1640 culture medium (purchased from Tianrun Shanda Reagent Co., Ltd.) at 37°C, 5% CO 2 After culturing in the incubator for 24h, use ConA (2mg / ml, purchased from Xiasi Biological Company); and anti-CD 3 (4mg / ml purchased from Shanghai Wowu Biotechnology Co., Ltd.) co-stimulated cells and then at 37 ° C, 5% CO 2 After continuing to cultivate in the incubator for 24 hours, the total RNA was extracted with the total RNA extraction kit of Promega Company, the operation was car...
Embodiment 2
[0103] Example 2: Construction of a series of hIL22 mutants
[0104] Construction of mutant 6M that mutated all six amino acids of the receptor binding site (DNNTDV) into alanine (Ala)
[0105] First pair of primers:
[0106] Reverse R2: 5'GAGAATTCATATGGCACCCATCAGCTCCCCA3'
[0107] Forward FM: 5'AGCTGCAGCTGCAGCTGCAGCCAAGCTAGCCTCCTT3'
[0108] Second pair of primers:
[0109] Reverse RM: 5'GCAGCTGCAGCTGCAGCTCGTCTCATTGGGGAGAAA3'
[0110] Forward F2: 5′ACGGATCCTCAAATGCAGGCATTTCT3′
[0111] The PCR reaction system is as follows:
[0112] pBV220-hIL22 1ul
[0113] Forward primer R2 (or RM) 10umol / l 2ul
[0114] Reverse primer FM (or F2) 10umol / l 2ul
[0115] 10×buffer 5ul
[0116] 2.5mmol dNTP 4ul
[0117] Pyrobest High Fidelity Enzyme 0.5ul
[0118] Add water to 50ul
[0119] In the first round of PCR reaction, two fragments of R2-FM and RM-F2 were obtained, and then the second round of PCR was performed using these two fragments as templates
[0120] R2-FM 1ul
[01...
Embodiment 3
[0134] Construction of mutant 2NM that changes Asn at positions 68 and 69 to Ala
[0135] First pair of primers:
[0136] Reverse R2: 5'GAGAATTCATATGGCACCCATCAGCTCCCCA3'
[0137] Forward FM: 5′GTCTGTAGCTGCATCAGCCAAGCTAGCCTC3′
[0138] Second pair of primers:
[0139] Reverse RM: 5'GCTGATGCAGCTACAGACGTTCGTCTCATT3'
[0140] Forward F2: 5′ACGGATCCTCAAATGCAGGCATTTCT3′
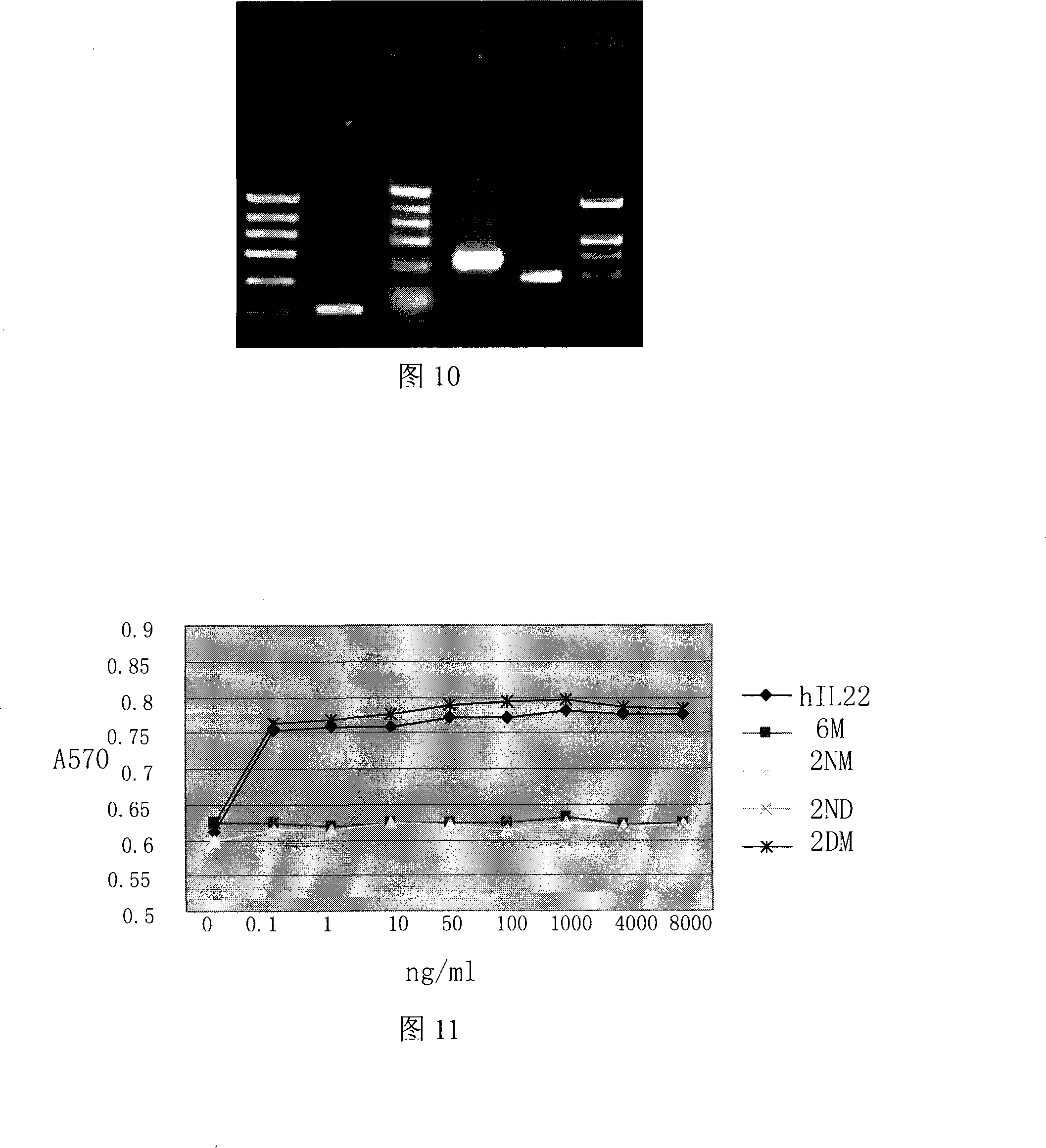
[0141] The reaction system and parameters are the same as before. The amplification result is shown in FIG. 8 , and the amino acid sequence is shown in SEQ NO:3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com