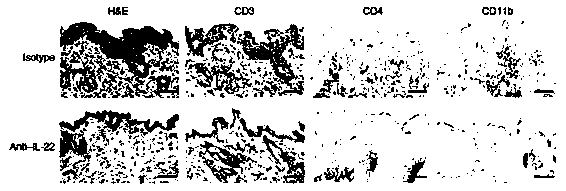
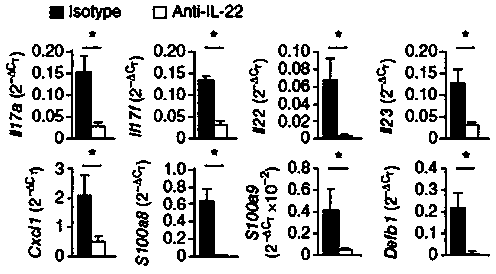
A kind of humanized anti-interleukin 22 genetic engineering antibody and its application
A humanized and antibody technology, applied in the field of biomedicine, can solve problems such as rapid clearance and ineffective activation of effector systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of human IL-22
[0038] Using genetic engineering technology to construct a human IL-22 gene expression vector (human IL-22 encoding nucleotide sequence includes SEQ ID NO. 7, and amino acid encoding sequence includes SEQ ID NO. 8). We constructed human IL-22 cDNA into pTXB 1 expression vector, transformed it into competent E. coli BL21 (DE3), prepared a single clone on LB (Amp+) agar plate, and cultured it overnight at 37°C. Transfer to 1L LB training center at the ratio of 1:100 the next day. Shake the bacteria at 37°C to A600=0.5~0.7, add IPTG (1mM) to induce expression. Shake at 37°C for 6-8 hours, centrifuge, and remove the supernatant. Resuspend the bacteria in 50ml Column buffer (Column buffer formula: 20 mM Tris-HCl, 1 mM EDTA, 1 mM DTT, pH 7.4 25°C), sonicate, and take the supernatant of the sonicated bacteria and pass through the column . Wash the column with 100ml Column buffer, and then wash it again with 30ml DTT buffer, 30mM (Tris-HC...
Embodiment 2
[0039] Example 2 Screening of humanized anti-IL-22 antibodies
[0040] 1. Screen positive antibodies from phage antibody library
[0041] Phage surface display technology is a new technology established and developed in recent years that uses phage to express foreign genes. It uses the restructured phage as a carrier, and inserts the candidate gene fragments into the gene region of the phage coat protein, so that the foreign polypeptide or protein is expressed and displayed on the phage surface, and then the specific peptide or protein expression is screened by affinity enrichment method. Phage. It realizes the conversion of genotype and phenotype, provides a high-efficiency screening system, and will have a profound impact in many basic and applied research fields. Because this technology has the potential to produce humanized antibodies, it has attracted many scholars to invest in this research, enabling the rapid development of phage antibody library technology, and thus creat...
Embodiment 3
[0047] Example 3 Preparation of recombinant antibodies
[0048] 1. Construction of recombinant antibody plasmid
[0049] The light chain of the obtained Fab antibody was cloned into the pAC-K-Fc vector (cat No. PROGENPR3003), and then the Fd segment of the heavy chain was cloned to construct a recombinant antibody expression vector.
[0050] 2. Transfection and determination of recombinant virus titer:
[0051] Transfect Sf9 cells (Pharmagen Baculo Gold co-transfection kit). For specific transfection methods, see the transfection instructions. After culturing at 27°C for 4-5 days, the supernatant was collected and the virus titer was determined.
[0052] 3. Secretory expression and purification of recombinant antibody IgG:
[0053] Sf9 cells were infected with virus, incubated at 27°C for 2 hours, replaced with SF-900 Ⅱ SFM serum-free medium, cultured at 27°C for 5 days, and the supernatant was collected. The supernatant was purified by affinity chromatography (Amersham, Protein-A affi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com