ELISA (Enzyme-linked Immunoassay Assay) plate and kit for predicting ALI (Acute Lung Injury)/ARDS (Acute Respiratory Distress Syndrome) and assessing prognosis of ALI/ARDS
An enzyme-linked immunoassay detection and prognosis technology, applied in measurement devices, instruments, disease diagnosis, etc., can solve the problem of not predicting the survival of ALI/ARDS, save manpower and time, improve work efficiency, and achieve accurate results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
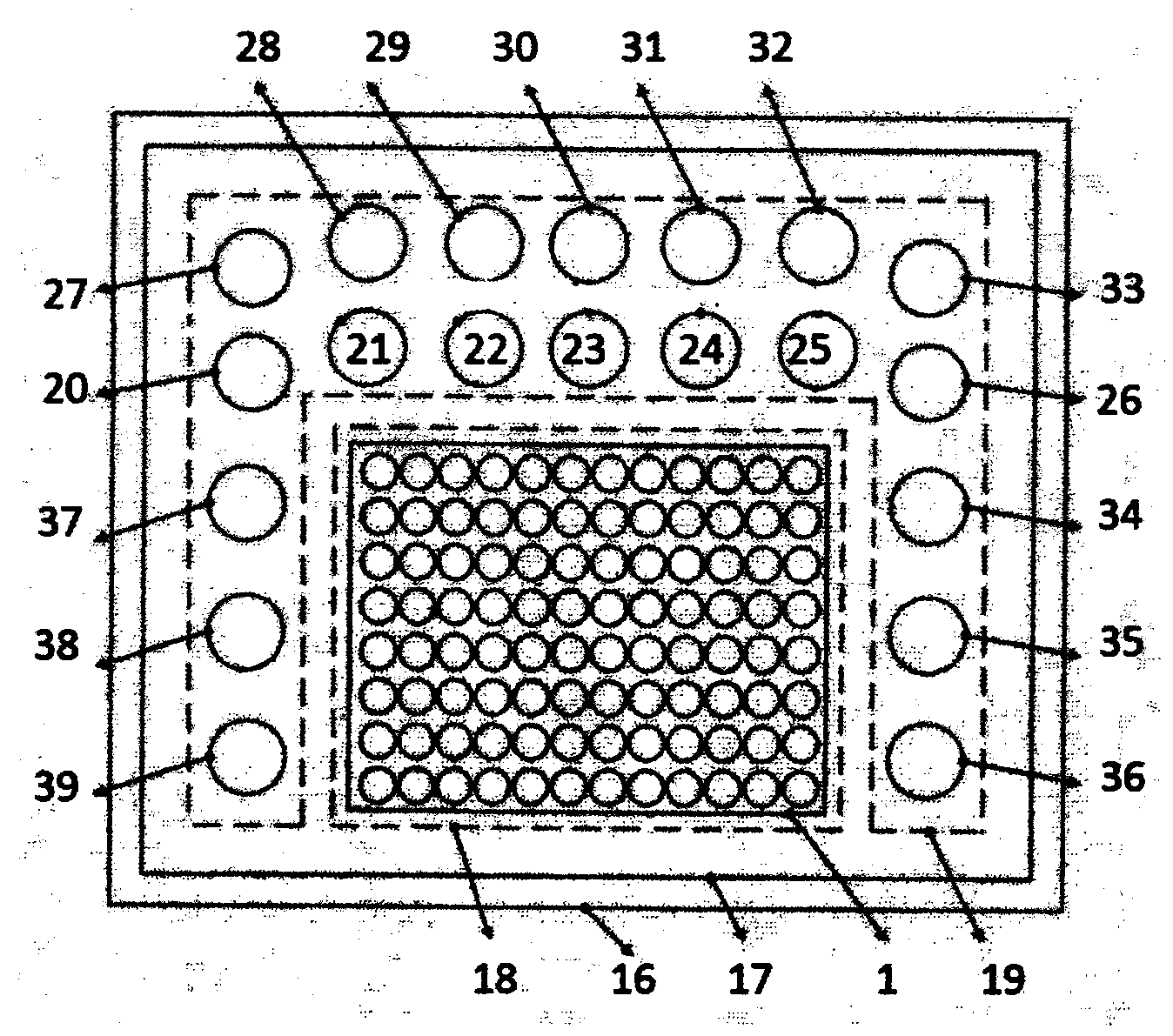
[0017] An enzyme-linked immunoassay panel for predicting ALI / ARDS and estimating its prognosis, comprising a solid phase carrier, on which a monoclonal antibody of bone morphogenic protein 15 (BMP-15), CXC chemotactic Monoclonal antibody to factor ligand 16 (CXCL16), monoclonal antibody to CXC chemokine receptor 3 (CXCR3), monoclonal antibody to interleukin-6 (IL-6), Wilms overexpressed gene (CCN3 / NOV ), monoclonal antibody to insulin-like growth factor binding protein 4 (IGFBP-4), monoclonal antibody to interleukin-5 (IL-5), monoclonal antibody to interleukin 5 receptor (IL-5R alpha) , monoclonal antibody to interleukin 22 receptor binding protein antibody (IL-22BP), monoclonal antibody to leptin (Leptin / OB), monoclonal antibody to macrophage inflammatory protein-1D (MIP-1d), hormone Monoclonal antibody to B (Orexin B), monoclonal antibody to CC class chemokine receptor 2 (CCR2), monoclonal antibody to transforming growth factor-beta5 (TGF-beta5), and blank control wells. Th...
Embodiment 2
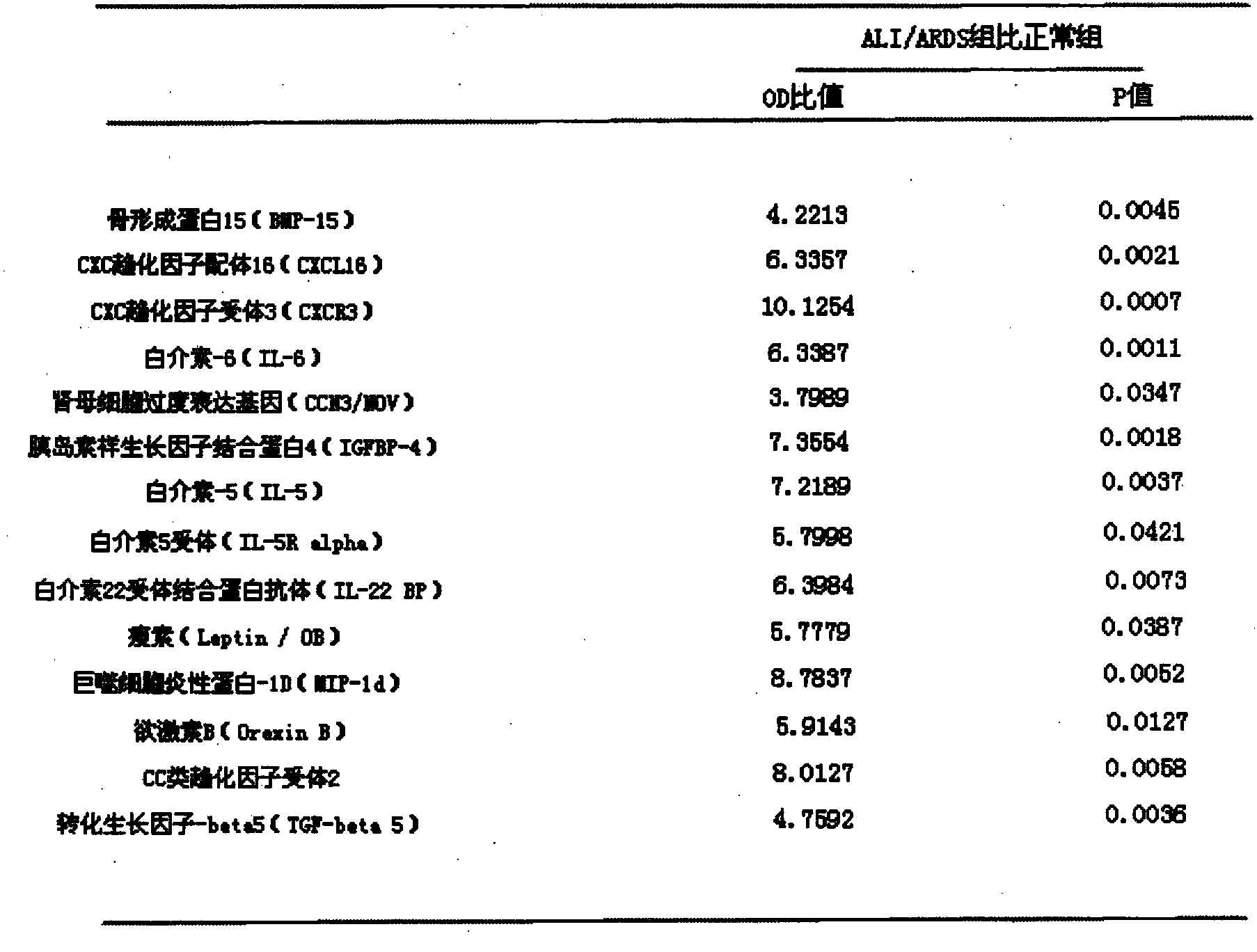
[0035] Collect 10 cases of normal human serum and 10 cases of ALI / ARDS patient serum as the normal group and ALI / ARDS group, use the kit in Example 1, and detect according to the detection steps in Example 1, the combined expression of 14 factors changes As shown in Table 1:
[0036] Table 1: Expression changes of 14 cytokines in ALI / ARDS patients and normal human serum
[0037]
[0038] As shown in Table 1, the OD ratios of the above 14 cytokines between the two groups were all higher than 3, and there were statistical differences in the OD values between the ALI / ARDS group and the normal group. The above results indicated that the 14 cytokines were significantly elevated in the serum of ALI / ARDS patients, so the simultaneous detection of the above 14 cytokines can be used as a clinical auxiliary means for the diagnosis and poor prognosis of ALI / ARDS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com