Extraction and purification process for recombinant protein
A recombinant protein and purification method technology, applied in the field of biopharmaceuticals, can solve the problems of low efficiency, low purity, and high production costs, and achieve the effects of improving purity and efficiency, increasing quantity and purity, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
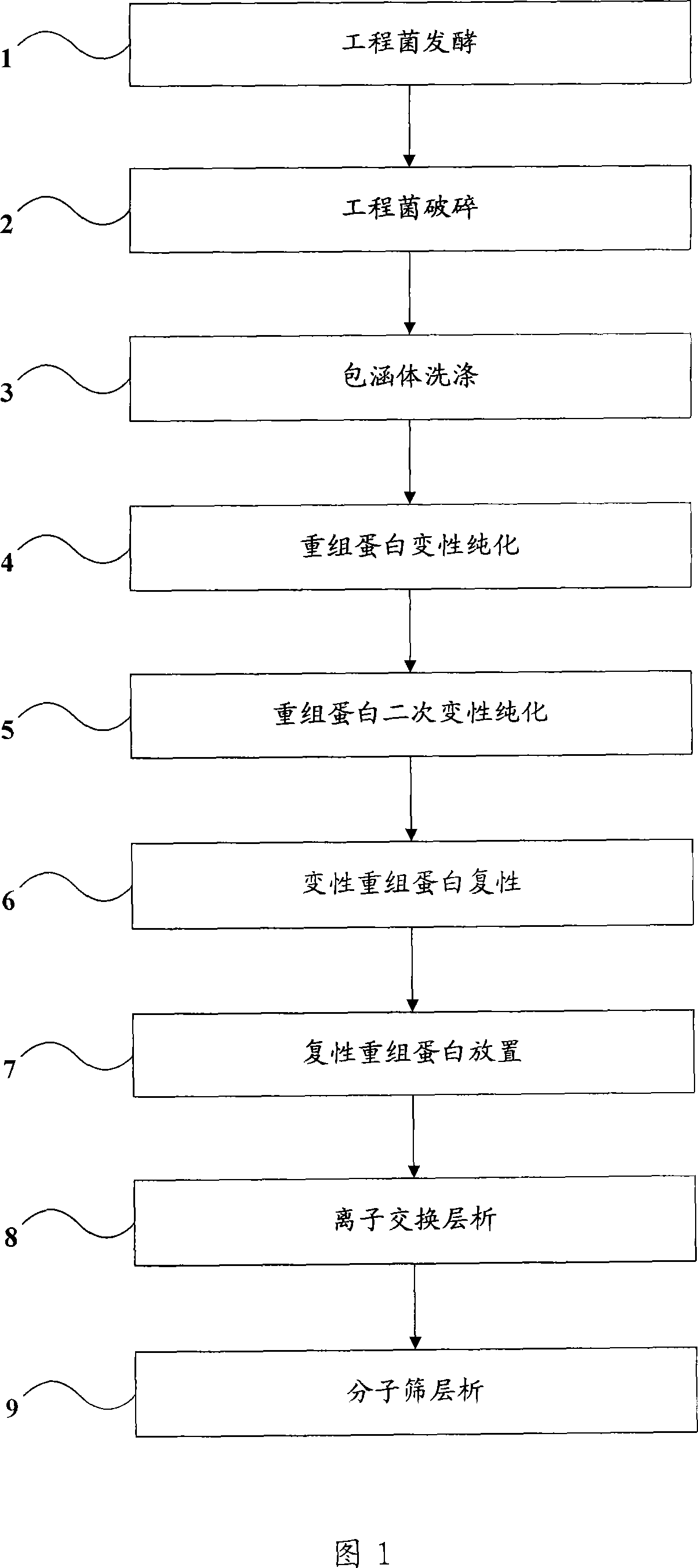
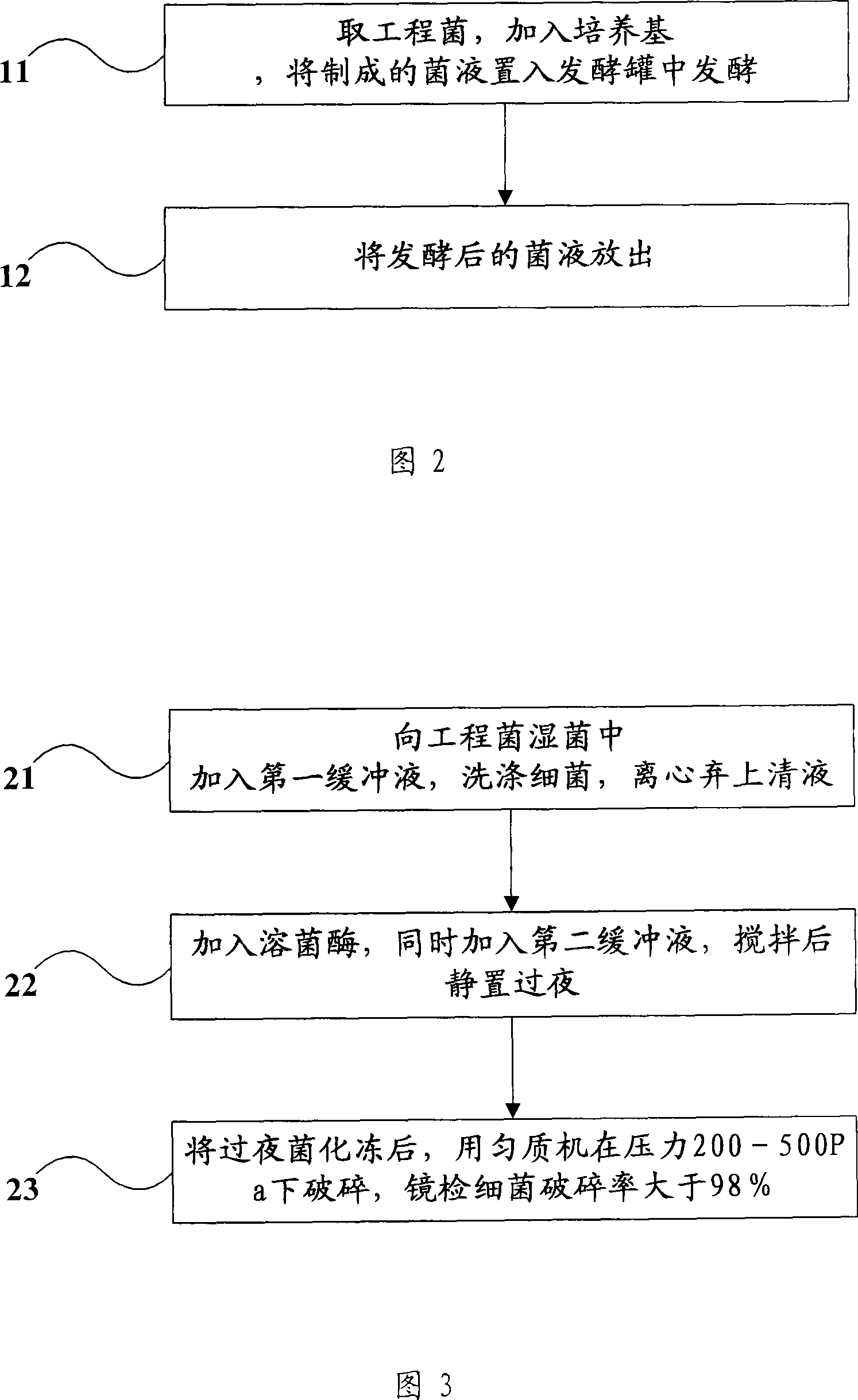
[0078] Step 101, get the engineering bacteria, carry out amplification with bacteriolysis broth medium (LB medium), put the engineering bacteria liquid of 10% of the fermentation volume into a fermenter containing high-density formula medium for fermentation, high-density formula The medium is 10g / L tryptone, 20g / L yeast extract, 12.5g / L hydrolase protein, 4g / L KH 2 PO 4 , 15g / L glucose;
[0079] Step 102, during the fermentation process, regularly and continuously drop increasing amounts of high-density formula culture medium;
[0080] Step 103, after 7.5 hours of fermentation, induce the engineered bacteria at a temperature of 42° C. for 3.5 hours, and release the fermented bacterial liquid from the fermenter after 11 hours of fermentation;
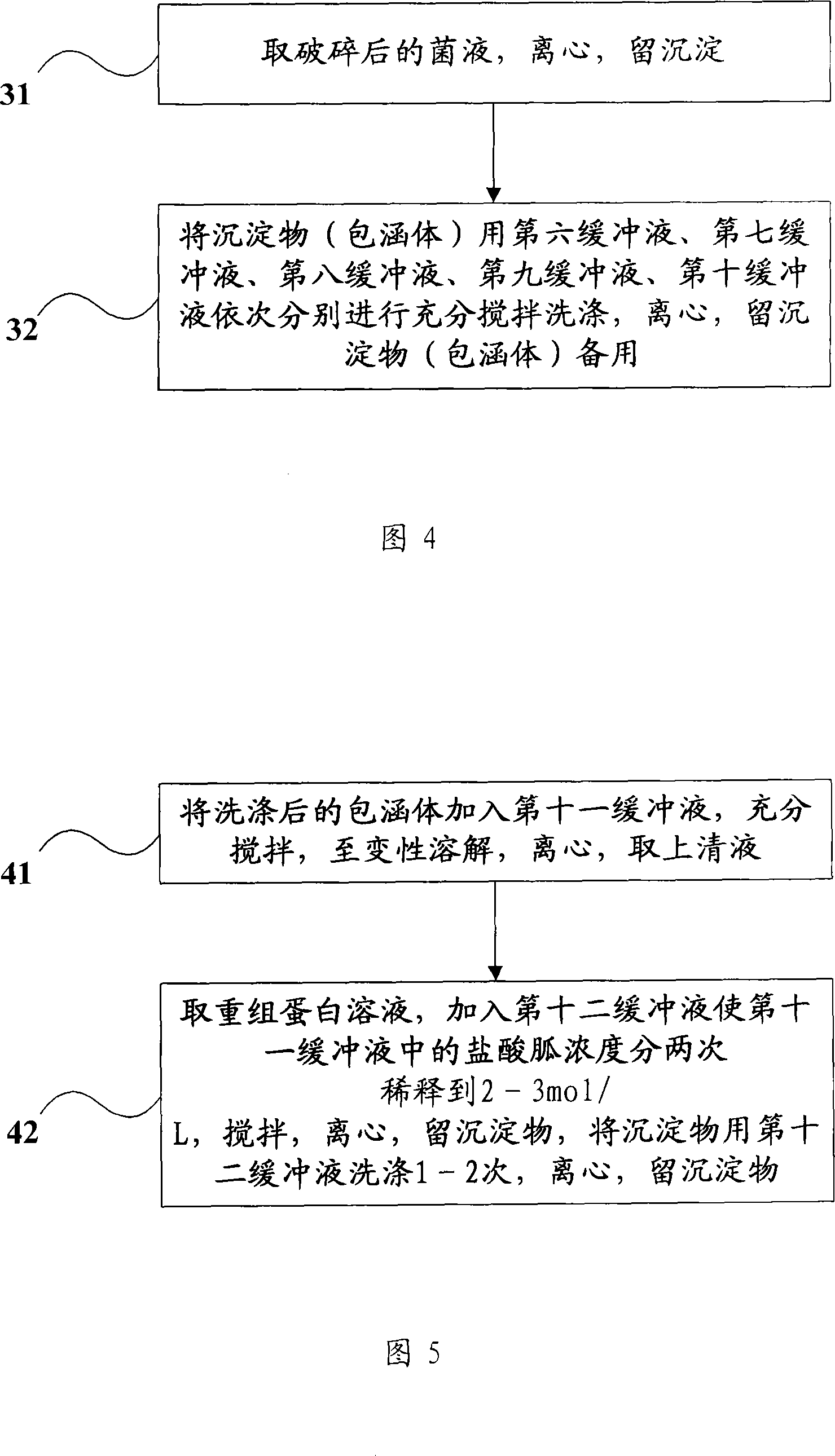
[0081] Step 104, take 100 g of engineering bacteria wet bacteria, add 1000 ml of the first buffer solution (20 mmol / LTris-HCl (tris-hydrochloric acid), pH 8.0), wash the bacteria, and centrifuge at 4 ° C for 10 minutes, Rotate at 700...
no. 2 example
[0098] Step 201, get engineering bacterium, after carrying out amplification with bacteriolysis broth culture medium (LB medium), insert the engineering bacterium of 10% by fermentation volume in the fermenter that contains high-density formula medium to carry out fermentation; High-density formula The medium is 5g / L tryptone, 15g / L yeast extract, 7.5g / L hydrolase protein, 1g / L KH 2 PO 4 , 10g / L glucose;
[0099] Step 202, during the fermentation process, regularly and continuously drop increasing amounts of high-density formula culture medium;
[0100] Step 203, after 8 hours of fermentation, inducing the engineered bacteria at a temperature of 42°C for 3 hours, and releasing the fermented bacterial liquid from the fermenter after 11 hours of fermentation;
[0101] Step 204, take 100 g of engineering bacteria wet bacteria, add 500 ml of the first buffer solution (20 mmol / LTris-HCl (tris-hydrochloric acid), pH 8.0), wash the bacteria, and centrifuge at 4 ° C for 10 minutes, ...
no. 3 example
[0118] Step 301, get the engineering bacteria, after amplifying with the bacteriolysis broth medium (LB medium), put the engineering bacteria liquid according to 10% of the fermented body into a fermenter containing a high-density formula medium for fermentation, high-density Formula medium is 15g / L tryptone, 25g / L yeast extract, 17.5g / L hydrolase protein, 9g / L KH 2 PO 4 , 20g / L glucose;
[0119] Step 302, during the fermentation process, regularly and continuously drop increasing amounts of high-density formula culture medium;
[0120] Step 303, after 7 hours of fermentation, inducing the engineered bacteria at a temperature of 42°C for 4 hours, and releasing the fermented bacterial liquid from the fermenter after 11 hours of fermentation;
[0121] Step 304, take 100 g of engineering bacteria wet bacteria, add 800 ml of the first buffer solution (20 mmol / LTris-HCl (tris-hydrochloric acid), pH 8.0), wash the bacteria, and centrifuge at 4 ° C for 10 minutes, Rotate at 7000rp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com