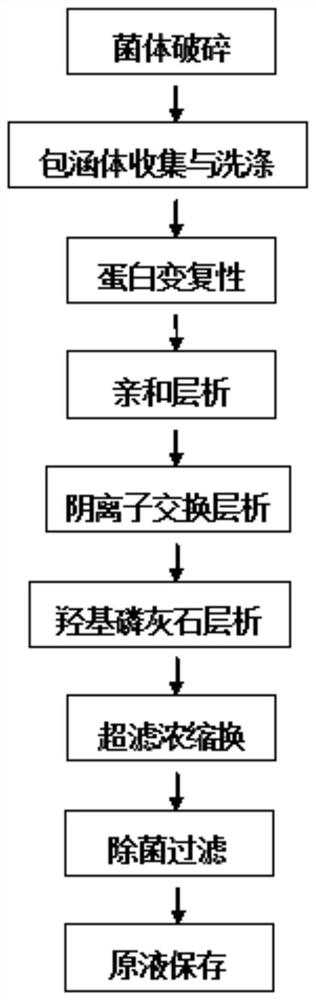
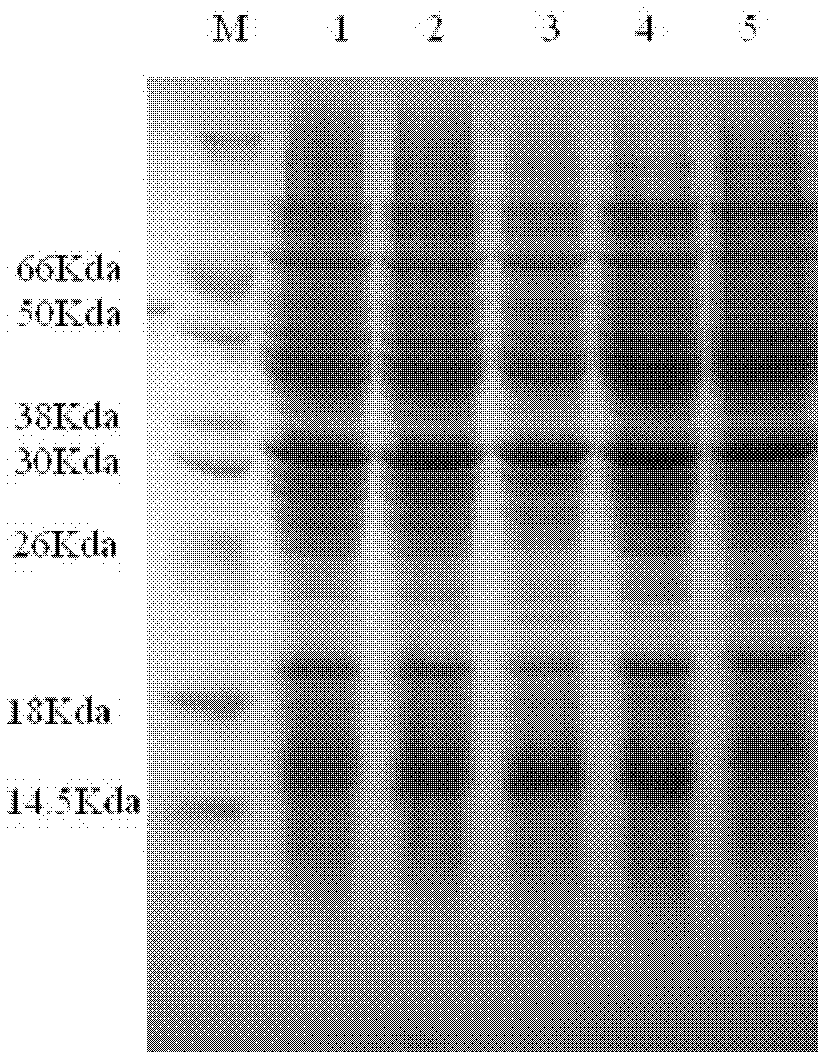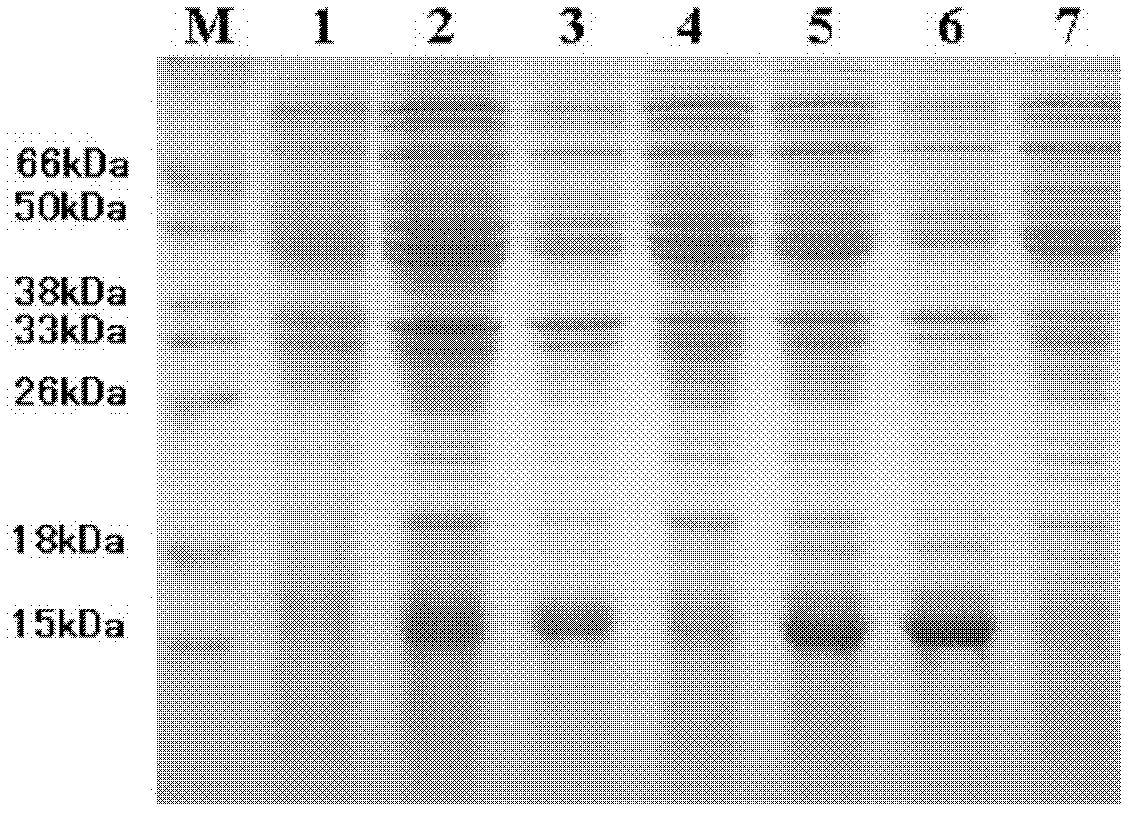Patents
Literature
34results about How to "Improve renaturation rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Renaturation liquid for recombining chicken alpha interferon inclusion body as well as preparation method and application thereof
InactiveCN102633875AReduce manufacturing costEasy to preparePeptide preparation methodsInterferonsEthylene diamineInclusion bodies
The invention provides a renaturation liquid for recombining a chicken alpha interferon inclusion body as well as a preparation method and an application thereof. The renaturation liquid mainly comprises arginine, EDTA (Ethylene Diamine Tetraacetic Acid), oxidized glutathione, Tris and glycerol, wherein the concentration of each component is as follows: 0.2-0.8 M of arginine, 0.5-2 mM of EDTA, 0.3-0.9 mM of toxidized glutathione, 60-100 mM of Tris and 15-30% (v / v) of glycerol; and the pH (Potential of Hydrogen) of the renaturation liquid is 8.0-8.5. The renaturation liquid is prepared by dissolving in distilled water and utilizing HCl to adjust the pH value; the renaturation liquid has an application prospect on the aspect of recombining the chicken alpha interferon inclusion body; and the renaturation liquid has a reasonable proportion for components to carry out a three-step washing and purifying and drip sample injection primary renaturation process method on the inclusion body to better separate a bacterial protein with the inclusion body, so that the renaturation rate of the protein is greatly improved.
Owner:TIANJIN SHENGJI GRP CO LTD
Production of specific micro-antibody for oarium cancer
InactiveCN1854295AHigh expressionImprove stabilityBacteriaImmunoglobulins against animals/humansEscherichia coliProcess optimization
The invention provides a nucleotide sequence encoding ovarian cancer anti-idiotypic microantibodies, a production method for efficiently producing ovarian cancer anti-idiotypic microantibodies, and related engineering cell construction, expression and purification processes. The optimized ovarian cancer anti-idiotypic microantibody gene is very suitable for expression in Escherichia coli. At the same time, by optimizing the combination of expression plasmids and host bacteria and optimizing the fermentation process, the expression level has been increased, and it has the advantages of high expression and high stability. The invention can obtain pure ovarian cancer anti-idiotypic micro-antibody with high efficiency, convenience and low cost.
Owner:SHANGHAI NEWSUMMIT BIOPHARMA
Freeze-dried protein renaturation method
InactiveCN101565448AAvoid damageReduce molecular thermal motionPeptide preparation methodsSolubilityFreeze-drying
The invention relates to a freeze-dried protein renaturation method based on a natural phenomenon that solute is naturally separated from solvent in the solution freezing process. The solubility of solute salt in a solution is influenced by temperature, denaturation salt (urea, guanidine hydrochloride, and the like) is gradually crystallized and separated from the solution because the solubility of the denaturation salt lowers at a low temperature, and two vital denaturation factors, namely molecular heat movement and the denaturation salt concentration of the solution, are reduced. The crystallization speed can be controlled by the temperature drop speed so as to establish a wonderful condition for protein renaturation, namely the low temperature as well as a smooth downward gradient of the denaturation salt concentration. Meanwhile, a renaturation solution is aided with antifreezing agents (DMSO, fucose, glucose, and the like) with a certain concentration, renaturation activators (cyclodextrin, glycin, molecular chaperones, and the like), reductant-oxidant, buffer salts, and the like for preventing freezing damage to the protein activity and improving the renaturation rate. The frozen dry protein is dried by a freeze drier to obtain solid particles which are suitably vibrated to break by utilizing the difference between the denaturation salt and dry protein powder in the crystallization proportion and the crystallization form and separated by adopting the methods of pneumatic separation, sieving, electrostatic adherence and the like to obtain target protein.
Owner:张鹏
Purification production method for recombinant human bone morphogenetic protein-2
ActiveCN104447977AReduce precipitationLow costBone-inducing factorOsteogenic factorInclusion bodiesFiltration
The invention discloses a purification production method for recombinant human bone morphogenetic protein-2 (rhBMP-2 protein). The method comprises the following steps: 1) lysing an inclusion body containing rhBMP-2 protein, so as to obtain an inclusion body lysate; 2) successively performing affinity chromatography and cation exchange chromatography on the inclusion body lysate for separation purification, so as to obtain a denatured recombinant human bone morphogenetic protein-2 solution; 3) performing renaturation on the denatured rhBMP-2 protein, so as to obtain a renatured rhBMP-2 protein crude product; and 4) successively performing affinity chromatography and gel filtration chromatography for separation and purification, so as to obtain a renatured rhBMP-2 protein pure product. By using the provided method for purifying rhBMP-2 protein, the prepared product has the purity of 95% or more and the protein yield of 9.4%, and the endotoxin and DNA residues both accords with biological medicine quality requirements. The method is suitable for industrial large-scale production. Also the method provides reference for research and production of same-kind recombinant protein medicines.
Owner:YANTAI ZHENGHAI BIO TECH
Preparation of recombinant human nerve growth factor ( rhNGF ) protein and renaturation solution
ActiveCN102628058AImprove renaturation rateEasy to operatePeptide preparation methodsAnimals/human peptidesEscherichia coliInclusion bodies
The present invention relates to the preparation of a recombinant human nerve growth factor (rhNGF) protein and a renaturation solution. The method includes steps of: expressing rhNGF protein by engineering Escherichia coli; breaking the engineering Escherichia coli bacteria expressing the rhNGF protein; centrifuging and collecting rhNGF protein inclusion body; carrying out denaturation dissolving on the inclusion body by using a denaturation system to form an inclusion body dissolved solution; adding a renaturation solution with a flow greater than 1080ml / min into the inclusion body dissolved solution for renaturation; and purifying the rhNGF protein. The renaturation solution contains 45-55mM Tris-HCl, 0-10mM beta- mercaptoethanol, 0-1mM cupric salt and 0.95-1.05mM EDTA, and has a pH of 8.5-10. The method of the invention utilizes instantaneous addition of renaturation solution for dilution and renaturation to enhance renaturation rate and reduce the production cost, and is simplefor operation.
Owner:ZHEJIANG NUPTEC BIOMACROMOLECULAR PURIFICATION RESINS MFG INC LTD
High-efficiency mixing renaturation device beneficial to rPA (radar Performance Analyzer) large-scale renaturation
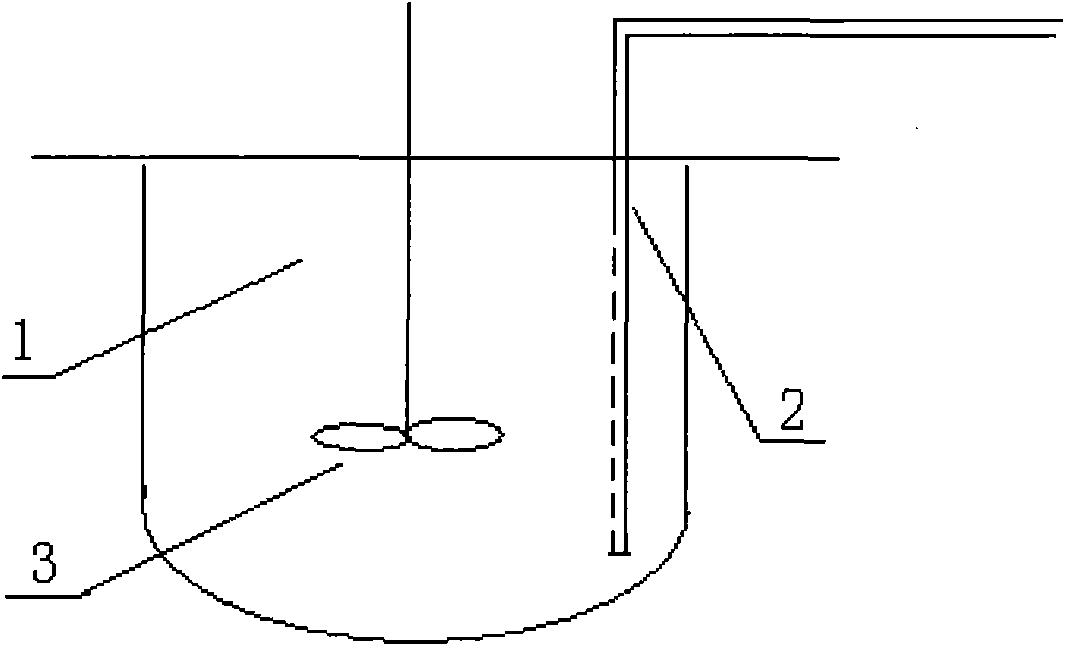
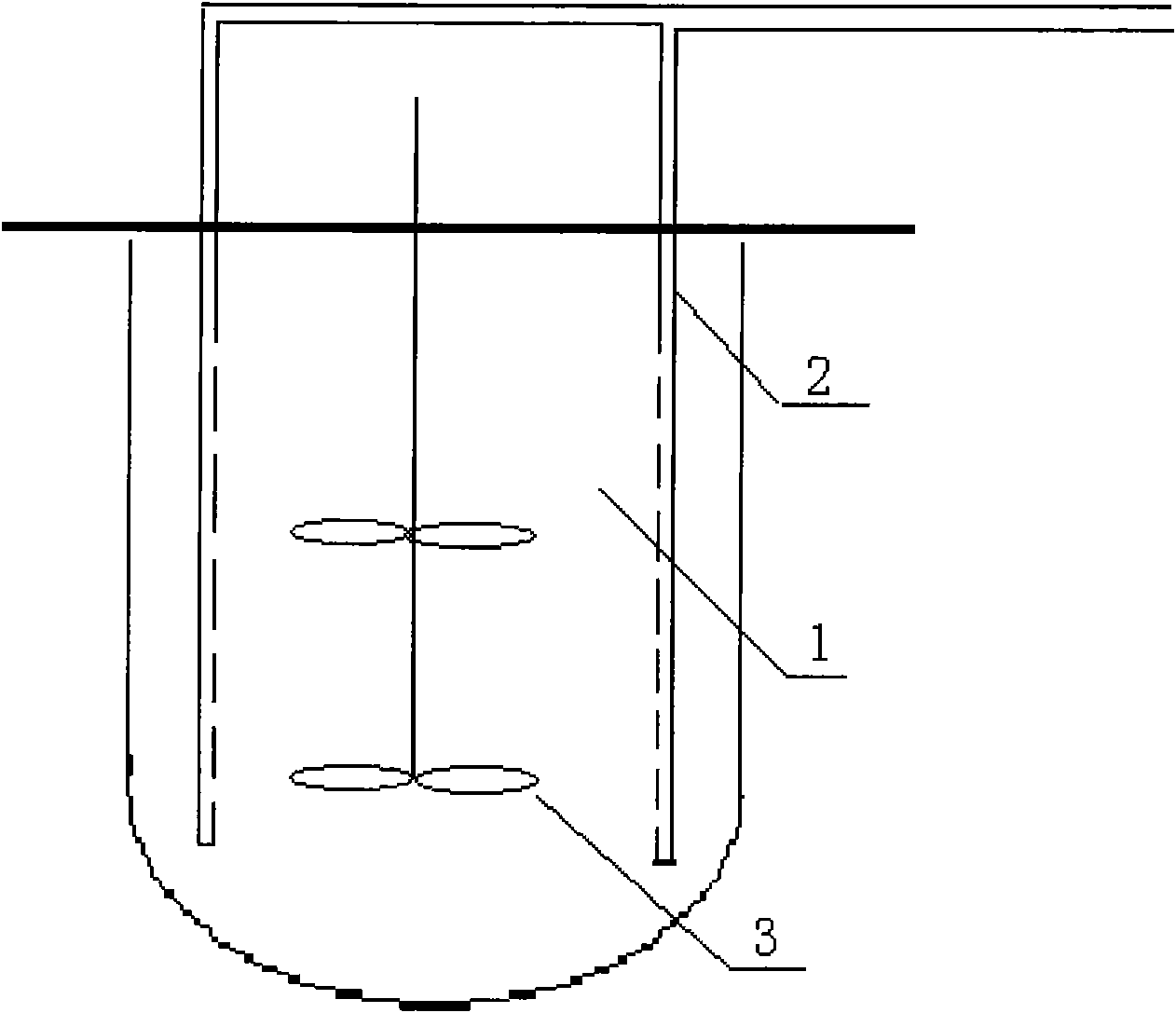
ActiveCN101831379AInhibition of aggregationConducive to large-scale industrial renaturationBioreactor/fermenter combinationsBiological substance pretreatmentsProtein solutionInclusion bodies
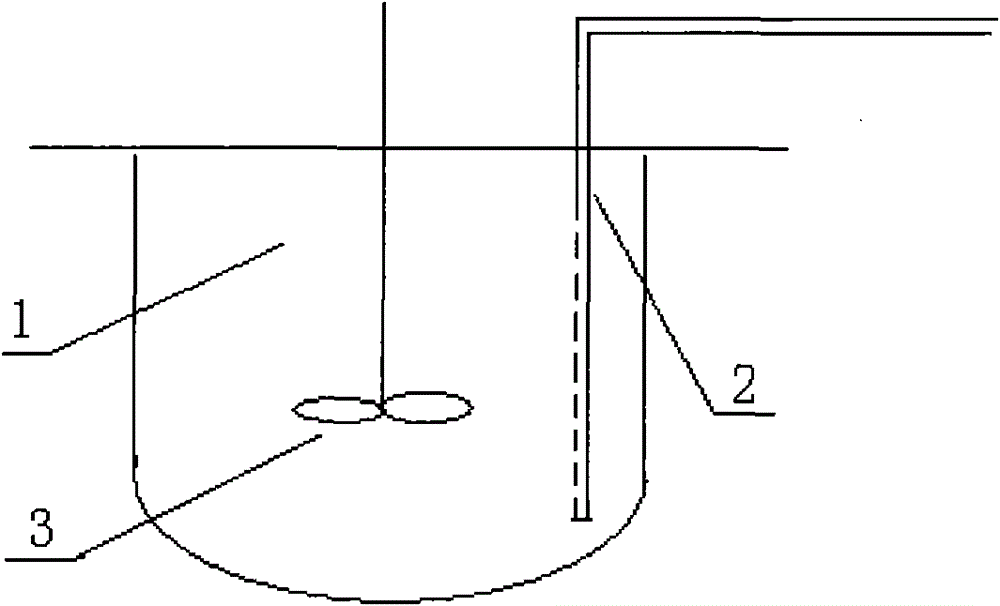
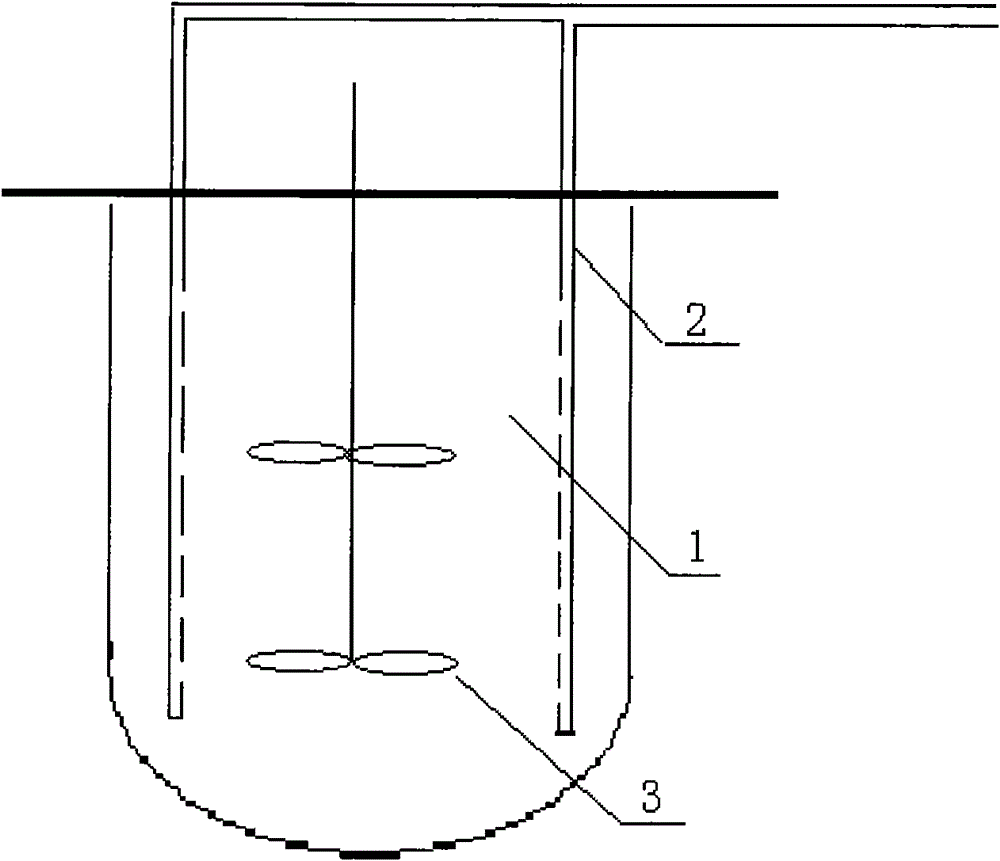
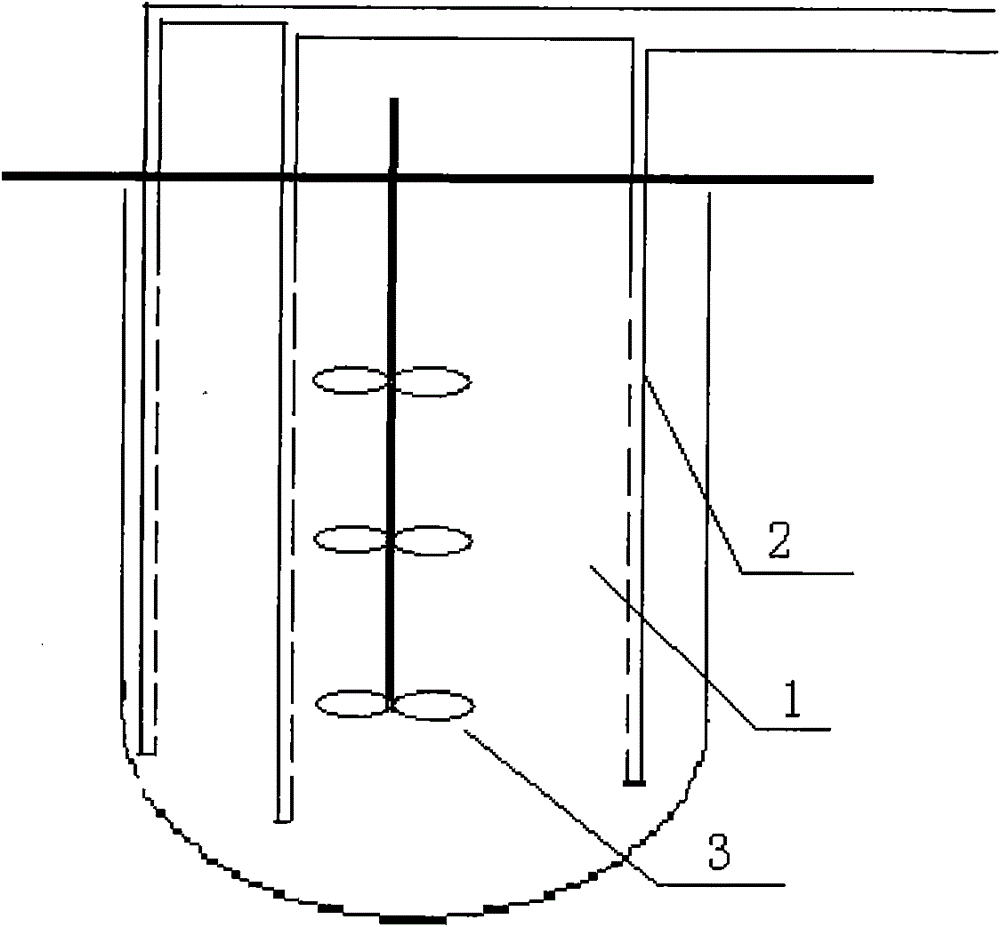
The invention relates to a high-efficiency mixing renaturation device which is beneficial to rPA large-scale renaturation and is used for adding a denatured protein solution into a renaturation buffer solution. The device comprises a container, a liquid inlet pipe and a propeller, wherein the renaturation buffer solution is loaded in the container; the liquid inlet pipe is provided with a plurality of nozzles, one end of the liquid inlet pipe is a liquid outlet end, the other end is a liquid inlet end, and the denatured protein solution is introduced into the liquid inlet end; and the propeller is inserted into a position below the liquid level of the renaturation buffer solution. Therefore, the invention can effectively inhabit the agglutination reaction of an rPA inclusion body when in renaturation and markedly improve the renaturation ratio of target protein.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Extraction and purification process for recombinant protein
ActiveCN101220082BNatural structural stabilityImprove renaturation ratePeptide preparation methodsMolecular sieveMicrobiology
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Method for purifying regrouped chicken colibacillosis outer membrane protein inclusion body and carrying out renaturation by utilizing high-efficiency renaturation liquid
InactiveCN104628832AImprove renaturation rateSimple equipment requirementsPeptide preparation methodsDepsipeptidesEscherichia coliMycoprotein
The invention belongs to the technical field of protein purification, and relates to the field of downstream protein purification of biological medicine and bioengineering, and discloses a method for purifying a regrouped chicken colibacillosis outer membrane protein inclusion body and carrying out renaturation by utilizing high-efficiency renaturation liquid. By adopting a three-step washing purification technological method of the inclusion body proteins, mycoproteins can be well separated from the inclusion body, the renaturation rate of the proteins can be greatly improved by virtue of a renaturation solution and a dropwise feeding one-step renaturation technological method, the renaturation process and needed equipment can be simplified, the yield rate of the target protein can be greatly increased, and the production cost of the regrouped chicken colibacillosis outer membrane protein can be reduced. The regrouped chicken colibacillosis outer membrane protein purification method is simple and easy, and the column-passing treatment is not needed.
Owner:TIANJIN SHENGJI GRP CO LTD
Renaturation method of restructured human insulin prokaryotic-fusion protein
ActiveCN105732820ASimple processing methodSimple and efficient operationAntibody mimetics/scaffoldsPeptide preparation methodsEscherichia coliHigh concentration
The invention relates to a renaturation method of restructured human insulin prokaryotic-fusion protein and belongs to the field of protein purification. The renaturation method includes steps of 1) smashing escherichia coli expressing restructured human insulin prokaryotic-fusion protein, and collecting inclusion bodies; 2) washing the inclusion bodies, dissolving the inclusion bodies and modifying the fusion protein; 3) renaturating protein. The renaturation method is needless of protein purification in advance, directly performs protein renaturation, and finally obtains high-concentration restructured insulin prokaryotic-fusion protein of natural structure. By the renaturation method, the defect of low protein concentration after protein renaturation and resultantly easy protein accumulation and precipitation is overcome, renaturation efficiency is up to 80-90%, production efficiency is improved, and the renaturation method is applicable to industrial production.
Owner:TONGHUA DONGBAO PHARMA
Method for highly efficient renaturation of membrane albumen
ActiveCN101054405AImprove renaturation rateAvoid damagePeptide preparation methodsInclusion bodiesProtein target
The invention discloses a high effectively membrane protein renaturation method, which comprise: purifying dissolved inclusion bodies protein in hydrophobic chromatography column, desalting using molecular sieve, carrying out denaturant concentration gradient elution and salt concentration gradient elution through ion exchange column chromatography, thus induction protein gradually folding right and renaturing . Any small molecule additive (as GSSG,GSH, PDI molecular chaperon etc.) is not employed in the complete process which reduce the cost. High uniformity, high activity target protein of above 98% in purity is obtained through the method which can be conveniently and continuously operated. The cost of the method is low.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Multi-subunit protein renaturation method
InactiveCN101343311AImprove renaturation rateIncrease productionPeptide preparation methodsComplete proteinProtein subunit
Disclosed is a refolding method of plural- subunits protein, which is characterized in that protein is composed of two or more than two subunits; one protein subunit or plural protein subunits is or are prerefolded firstly, and the other protein subunit or other plural protein subunits is or are combined on a chromatographic column; renaturation solution containing the prerefolded protein subunits is increased by step so that the protein subunits combined on the chromatographic column can be refolded and can be combined with the prerefolded protein subunits to form a complete protein molecule; and after being washed, the refolded protein is eluted from the chromatographic column. With the refolding method to prepare the protein composed of plural subunits, the yield of the protein is high, the protein is not needed to be purified, the operation is simple, and the raw material, the time and the labor can be saved.
Owner:SOUTHEAST UNIV
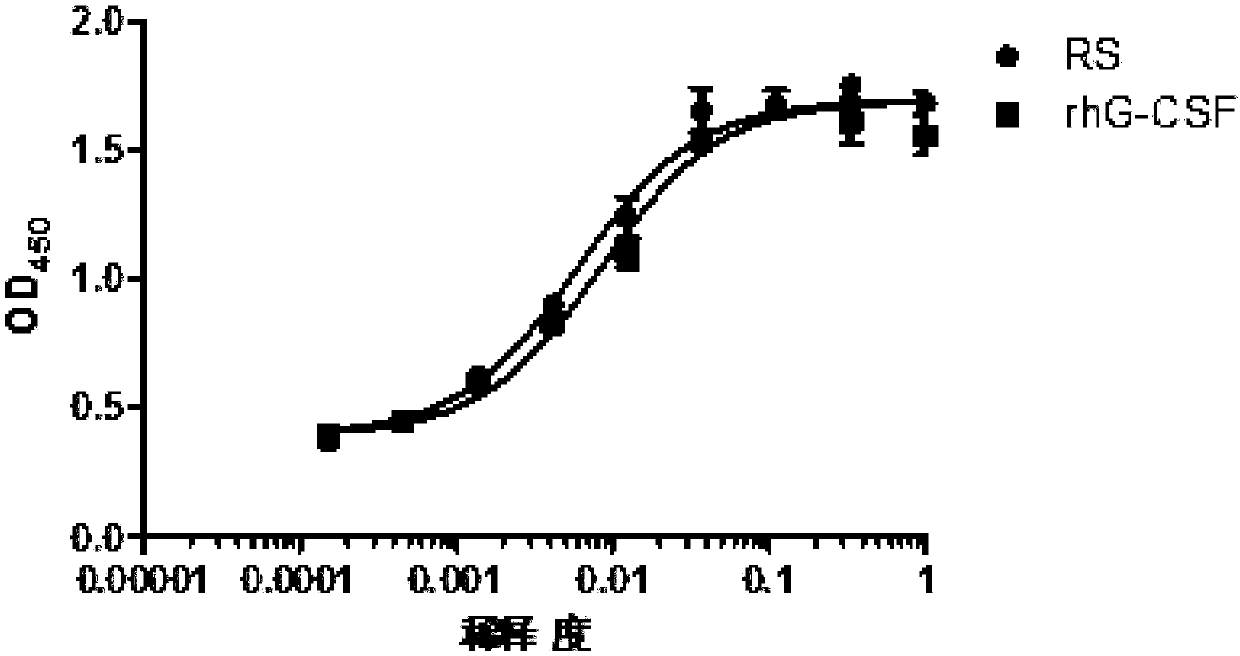
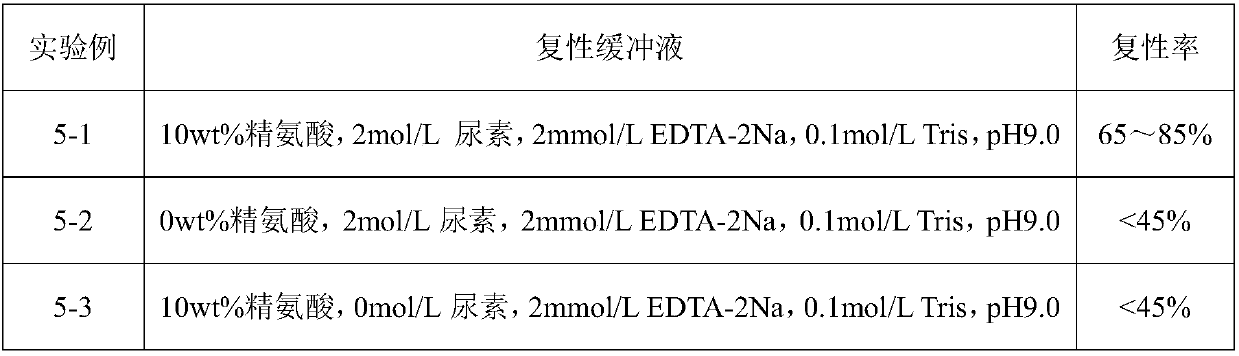
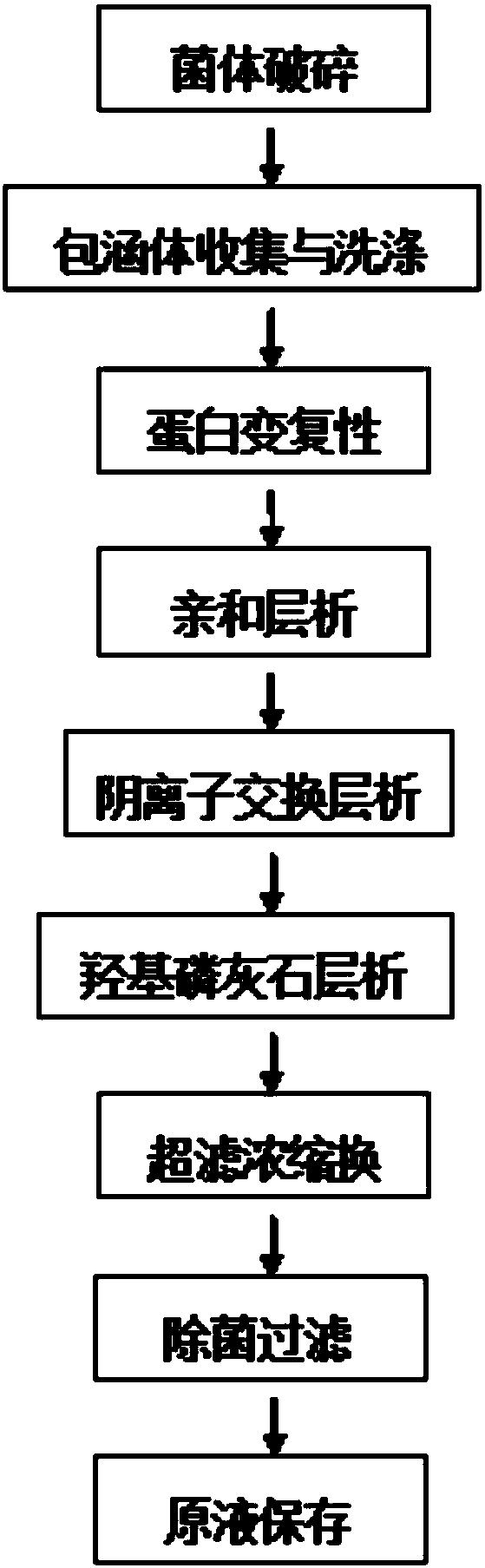
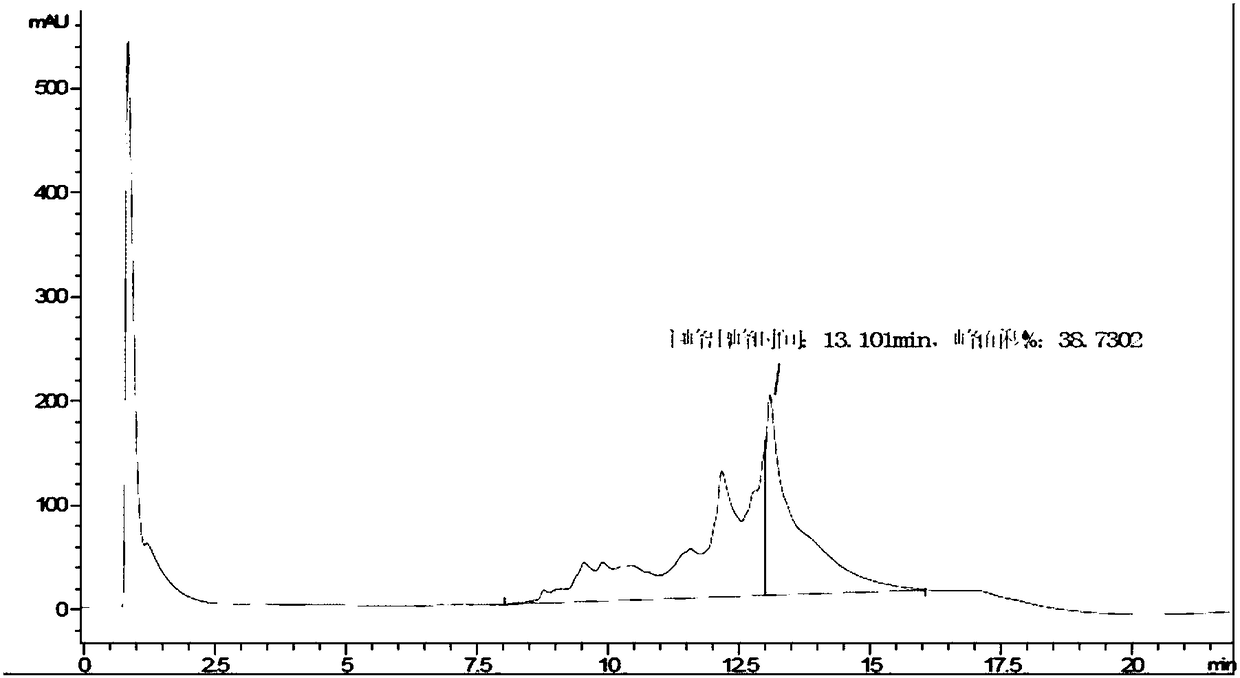
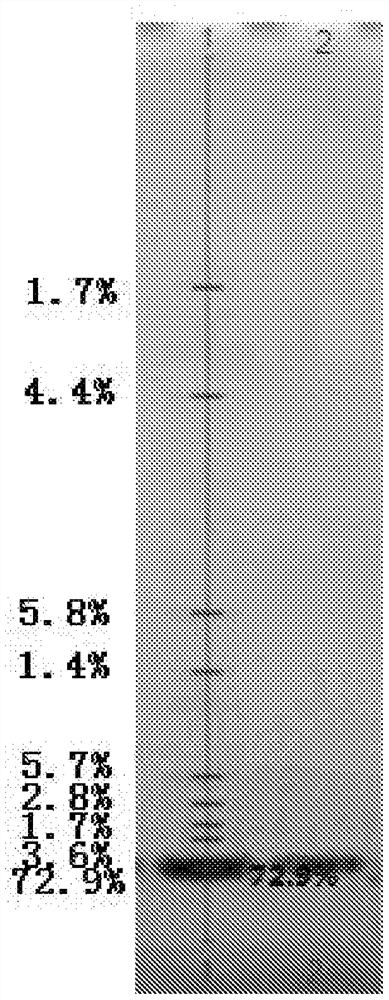
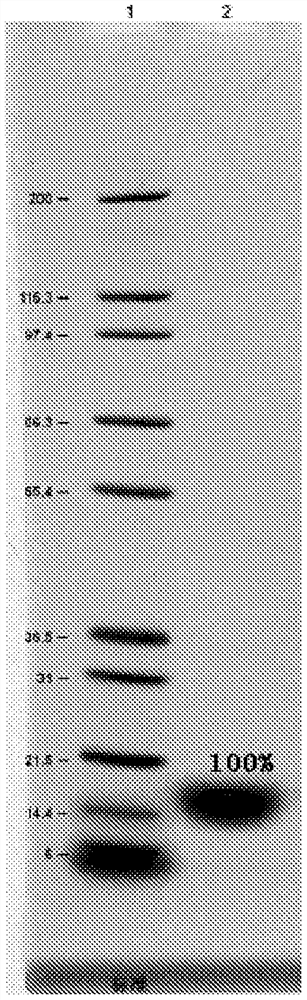
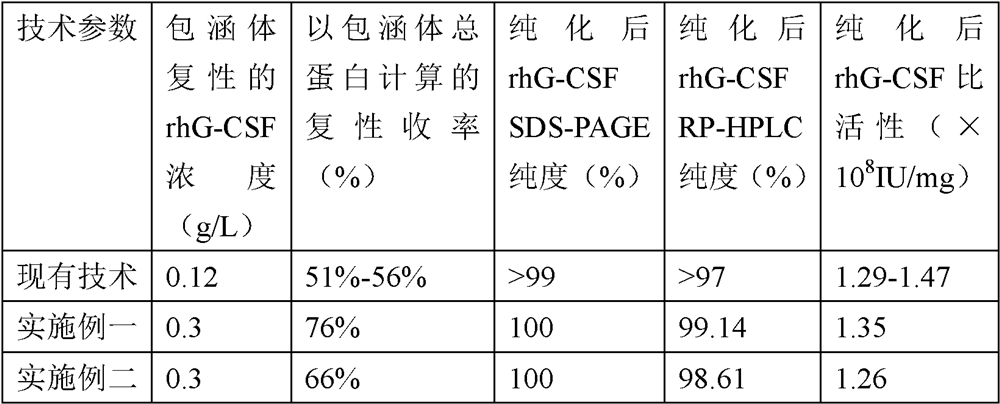
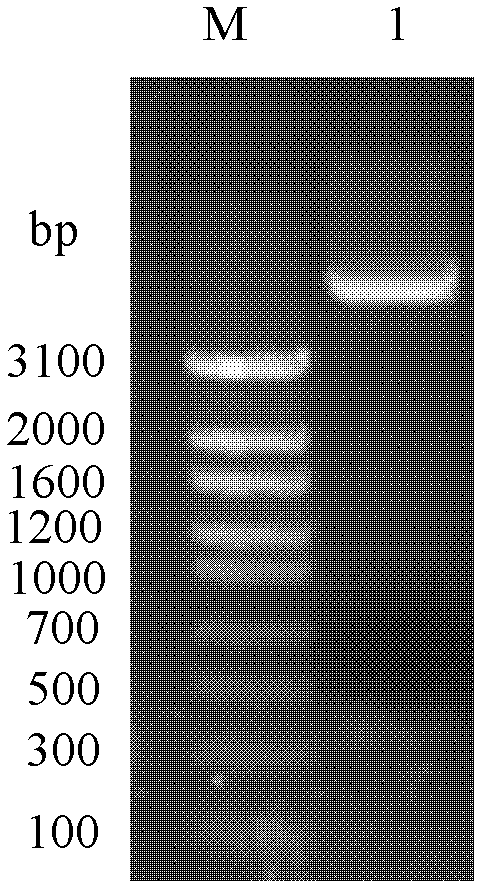
Renaturation and purification method of recombinant human granulocyte colony stimulating factor
InactiveCN110041423ASimple and fast operationShort processing timePeptide preparation methodsDepsipeptidesInclusion bodiesPurification methods
The invention provides a renaturation and purification method of a recombinant human granulocyte colony stimulating factor. The renaturation and purification method comprises the steps of (1) preparing inclusion body dissolving liquid; (2) adding the inclusion body dissolving liquid to renaturation buffer liquid, adding cystine and cysteine, and performing renaturation; and (3) applying the obtained renaturation liquid to a weak cation chromatography column with a composite ligand for purification. The purification technology is simple and convenient to operate, short in consumption time, andlow in equipment requirement; the cost is saved, and the purification technology is suitable for amplified production scale; and besides, during renaturation, inclusion protein bodies are high in concentration and high in renaturation rate, and after chromatography purification, protein purity is high.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for purifying proteins and kit
ActiveCN108264547AHigh removal rateImprove renaturation rateThrombopoietinPeptide/protein ingredientsInclusion bodiesFermentation
The invention belongs to the field of biochemistry and fermentation engineering, and relates to a method for purifying proteins and a kit. Particularly, the method for purifying the proteins comprisesthe step that chromatography is carried out on refolding products of protein inclusion bodies. The method for purifying the proteins has the advantages that the renaturation rate is high, the yield is high, and the application prospect is good.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
A cyclic chromatography renaturation method for recombinant human bone morphogenetic protein-2
ActiveCN104961820BReduce consumptionLow costBone-inducing factorOsteogenic factorInclusion bodiesBone morphogenetic protein 6
The invention discloses a recombinant human bone morphogenetic protein-2 recycling chromatography renaturation method. The method comprises 1, carrying out denaturation on a human bone morphogenetic protein-2 inclusion body to obtain a denatured protein, and carrying out affinity chromatography renaturation on the denatured protein, and collecting the eluent to obtain the recombinant human bone morphogenetic protein-2 dimer subjected to renaturation. The human bone morphogenetic protein-2 has an amino acid sequence shown in the formula 2 in the sequence table. An experiment proves that the method realizes rhBMP-2 protein renaturation, has the advantages of high protein concentration, less reagent consumption and renaturation rate of 70% or more, is suitable for industrial large-scale production and provides reference for research and development of the same type of a recombinant protein drug.
Owner:YANTAI ZHENGHAI BIO TECH
Method for preparing protein inclusion body and recombinant human beta-neural growth factor
PendingCN110093394AImprove renaturation rateShorten the refolding timePeptide preparation methodsFermentationProtein proteinInclusion bodies
The invention relates to a method for preparing a protein inclusion body and a recombinant human beta-neural growth factor. According to the invention, the inclusion body of the recombinant human beta-neral growth factor precursor protein containing the enterokinase enzyme sites is subjected to chromatographic renaturation to obtain a renaturation precursor protein, which is the protein inclusionbody; the renaturation precursor protein is digested with enterokinase to obtain an enzymatic hydrolysate; and the enzymatically hydrolyzed product is isolated and purified. The method applies a chromatographic method to the renaturation of the rhpro-NGF inclusion body, and significantly improves the renaturation rate and shortens the renaturation time compared with the traditional dilution renaturation method, so that the subsequent purification is simpler, and a high-purity product having a correct structure is obtained, the method can reduce the chance of protein aggregation, breaks the balance of complex denaturing reaction, and makes the renaturation reaction continue, is carried out with high protein renaturation concentration, is convenient for removing a denaturing agent, integrates renaturation and purification processes, and can realize automation and scale, and a chromatographic medium can be reused, which reduces the process cost and is more conducive to industrialized massproduction in the future.
Owner:重庆科润生物医药研发有限公司
Purification method of tumor vessel blocking agent fusion protein
ActiveCN107400169ABiological function is not lostImprove biological activityPeptide/protein ingredientsPeptide preparation methodsEscherichia coliProtein target
The invention provides a purification method of tumor vessel blocking agent fusion protein. The method comprises the steps of smashing a fermentation biomass of the tumor vessel blocking agent fusion protein expressed by escherichia coli and existed in an inclusion body way through a high pressure homogenizer, and carrying out high speed centrifugation to obtain a coarse inclusion body; washing through different washing solutions, and obtaining a cleaner inclusion body; then carrying out degeneration and dissolution on the inclusion body through 8M urea, and centrifuging to obtain a supernatant; using a dialysis method for carrying out renaturation; after renaturation, carrying out ion exchange and molecular sieve column purification on a sample, and obtaining the target protein with SDS-PAGE electrophoresis purity and RP-HPLC purity reaching to 95 percent or above. The purification method provided by the invention is simple and convenient, low in cost, easy to operate, and easy in industrialization production.
Owner:BEIJING HUAAN INNOVATION BIOTECH
Recombinant protein renaturation buffer solution as well as preparation method and application thereof
InactiveCN103626834ALess volume increaseConcentration dilution is smallPeptide preparation methodsBuffer solutionBeta-Cyclodextrins
The invention relates to a recombinant protein renaturation buffer solution as well as a preparation method and an application thereof. The buffer solution consists of a basis solution, 0.5% of beta-mercaptoethanol, 1% of tween-20, 10mM of beta-cyclodextrin, 1M of L-cysteine and 4M of urea. The preparation method comprises the following steps: firstly, adding 4M of urea into the basis solution, secondly, adding 0.5% of beta-mercaptoethanol, 1% of tween-20, 10mM of beta-cyclodextrin and 1M of L-cysteine according to volume ratio, so as to form the recombinant protein renaturation buffer solution. The buffer solution provided by the invention is applied to recombinant protein renaturation with the primary protein concentration greater than 1mg / mL, is simple to operate, low in cost and convenient in industrial production, the volume after renaturation is slightly increased, the protein concentration is slightly diluted, and the renaturation rate is high.
Owner:JIANGSU ACAD OF AGRI SCI
Food-grade lactococcus lactis with human metallothionein-I anchored on surface and preparation method and use thereof
ActiveCN104818227AEliminate the cumbersome steps of separation and purificationReduce lossBacteriaWater contaminantsEscherichia coliThionin
Owner:SOUTH CHINA UNIV OF TECH
Refolding and purification method of recombinant human granulocyte stimulating factor
ActiveCN108368162BOptimize the washing processAdded steps to remove reducing agentPeptide preparation methodsDepsipeptidesSide effectMedicine
A method for renaturation and purification of recombinant human granulocyte-stimulating factor, which improves the controllability and renaturation rate of the renaturation process by removing the reducing agent by buffer replacement before renaturation, and optimizes the concentration of the renaturation buffer composition. The above-mentioned method is suitable for large-scale industrial scale-up production, has low requirements on equipment, simple operation, high purity and good stability of the obtained protein, can reduce the side effects of the final product in clinical practice, and improve the safety and effectiveness of medicines.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Preparation of recombinant human nerve growth factor ( rhNGF ) protein and renaturation solution
ActiveCN102628058BImprove renaturation rateEasy to operatePeptide preparation methodsAnimals/human peptidesEscherichia coliInclusion bodies
The present invention relates to the preparation of a recombinant human nerve growth factor (rhNGF) protein and a renaturation solution. The method includes steps of: expressing rhNGF protein by engineering Escherichia coli; breaking the engineering Escherichia coli bacteria expressing the rhNGF protein; centrifuging and collecting rhNGF protein inclusion body; carrying out denaturation dissolving on the inclusion body by using a denaturation system to form an inclusion body dissolved solution; adding a renaturation solution with a flow greater than 1080ml / min into the inclusion body dissolved solution for renaturation; and purifying the rhNGF protein. The renaturation solution contains 45-55mM Tris-HCl, 0-10mM beta- mercaptoethanol, 0-1mM cupric salt and 0.95-1.05mM EDTA, and has a pH of 8.5-10. The method of the invention utilizes instantaneous addition of renaturation solution for dilution and renaturation to enhance renaturation rate and reduce the production cost, and is simplefor operation.
Owner:ZHEJIANG NUPTEC BIOMACROMOLECULAR PURIFICATION RESINS MFG INC LTD
High-efficiency mixing renaturation device beneficial to rPA (radar Performance Analyzer) large-scale renaturation
ActiveCN101831379BInhibition of aggregationConducive to large-scale industrial renaturationBioreactor/fermenter combinationsBiological substance pretreatmentsProtein solutionInclusion bodies
The invention relates to a high-efficiency mixing renaturation device which is beneficial to rPA large-scale renaturation and is used for adding a denatured protein solution into a renaturation buffer solution. The device comprises a container, a liquid inlet pipe and a propeller, wherein the renaturation buffer solution is loaded in the container; the liquid inlet pipe is provided with a plurality of nozzles, one end of the liquid inlet pipe is a liquid outlet end, the other end is a liquid inlet end, and the denatured protein solution is introduced into the liquid inlet end; and the propeller is inserted into a position below the liquid level of the renaturation buffer solution. Therefore, the invention can effectively inhabit the agglutination reaction of an rPA inclusion body when in renaturation and markedly improve the renaturation ratio of target protein.
Owner:EAST CHINA UNIV OF SCI & TECH +1
A food-grade Lactococcus lactis with surface-anchored human I-type metallothionein and its preparation method and application
ActiveCN104818227BEliminate the cumbersome steps of separation and purificationReduce lossBacteriaWater contaminantsBiotechnologyEscherichia coli
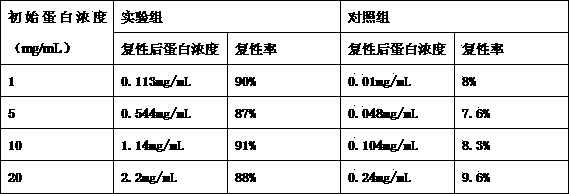
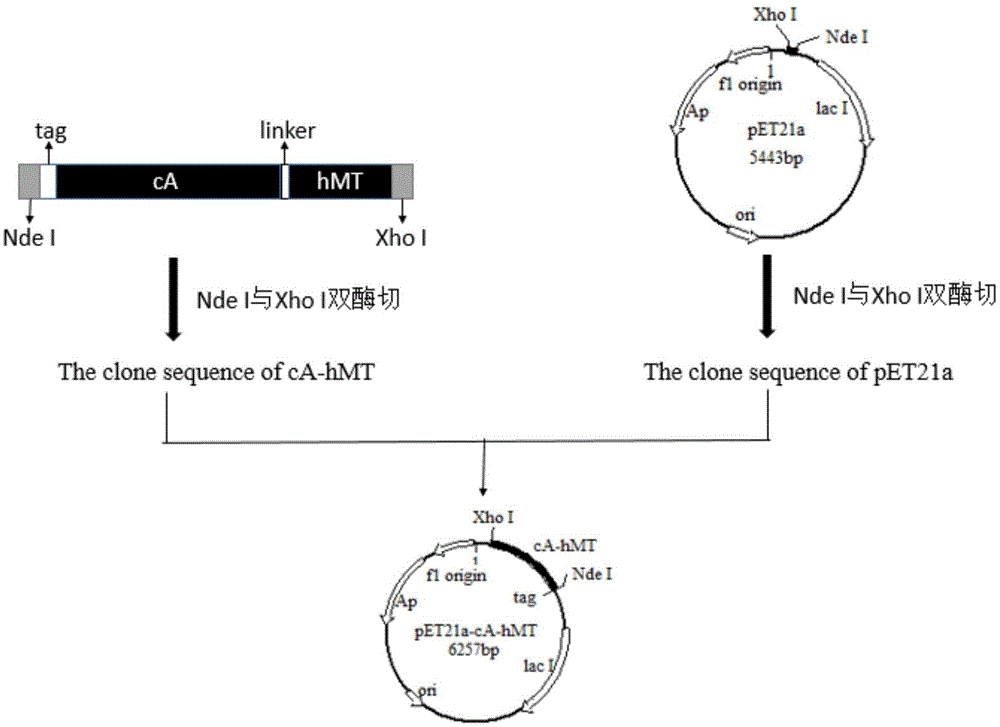
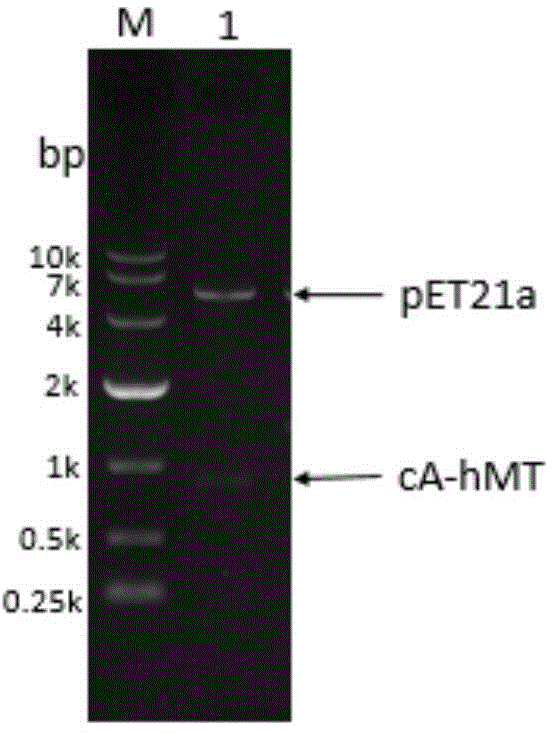
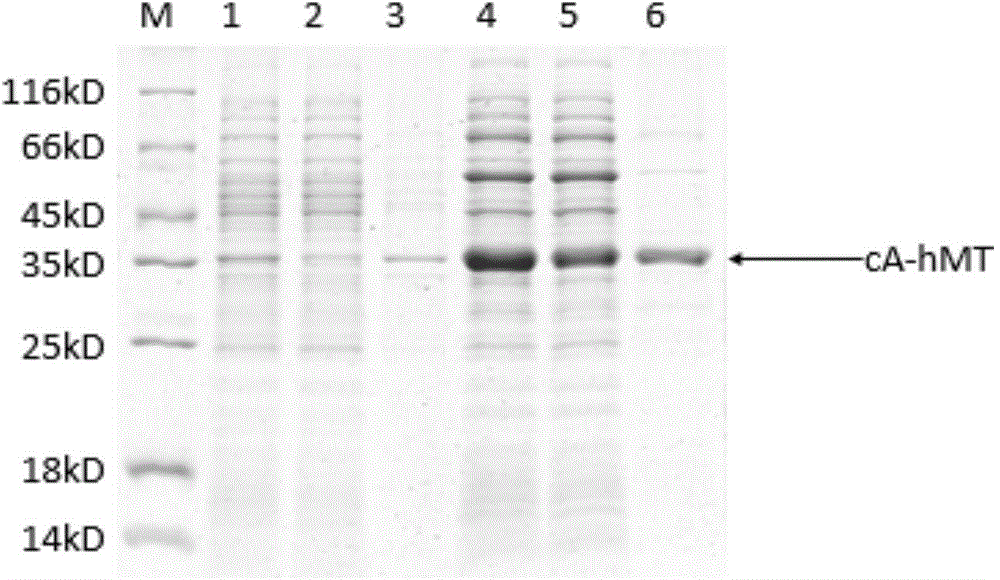
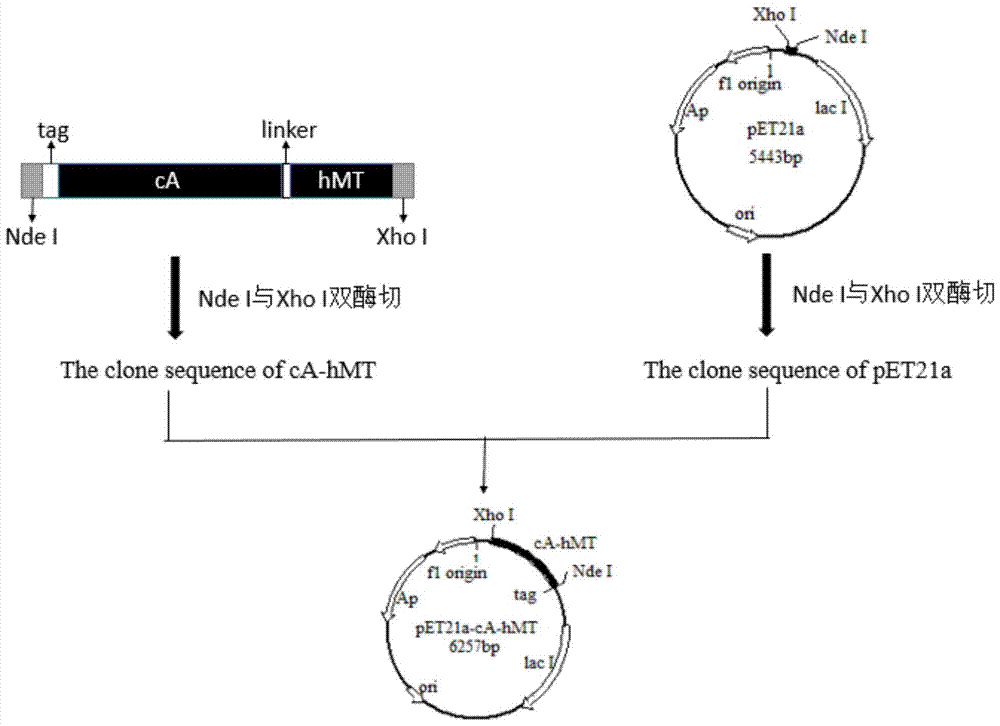
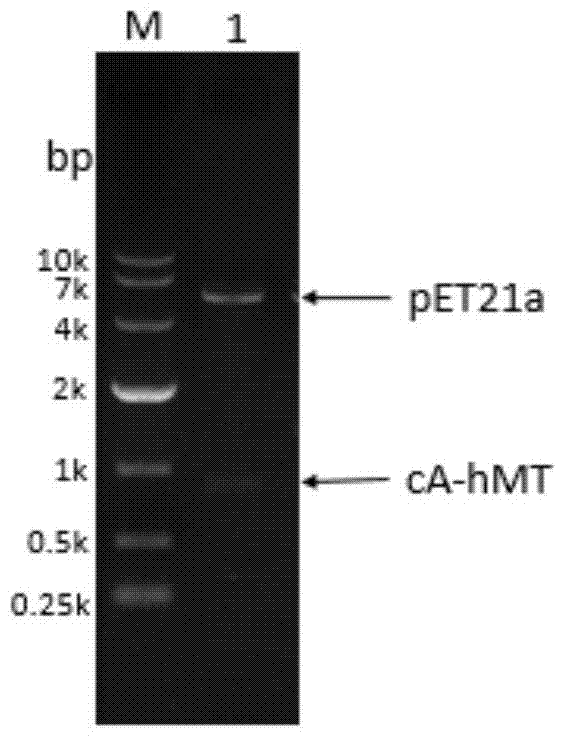
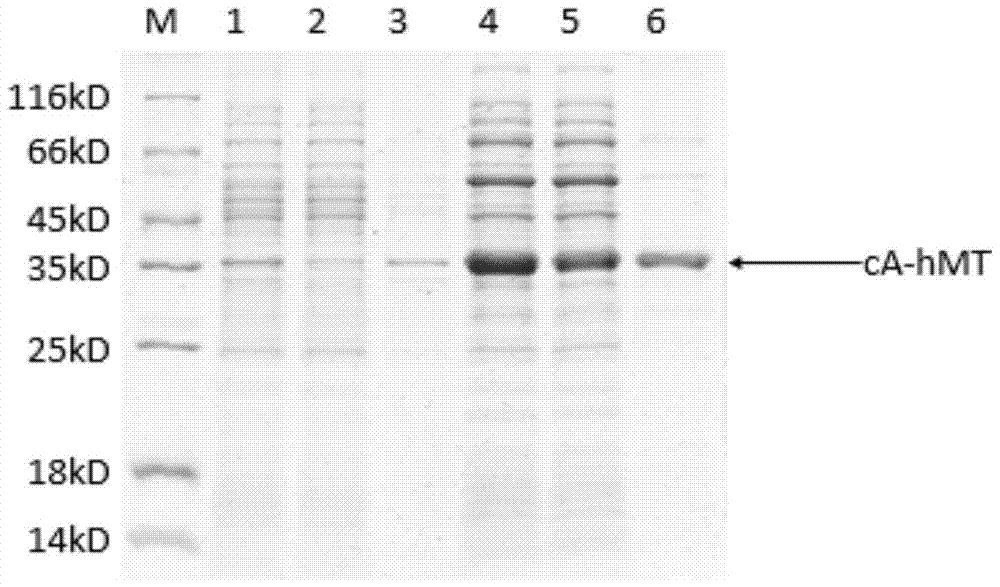
The invention relates to food-grade lactococcus lactis with human metallothionein-I anchored on the surface and a preparation method and a use thereof. The preparation method comprises the following steps of 1, splicing a cA gene and an hMT gene by overlapping PCR, introducing linker between the two genes, and introducing an expression promotion label to an end N to obtain a recombinant cA-hMT gene, 2, connecting the recombinant cA-hMT gene and an expression carrier to obtain a connection product and transferring the connection product into a escherichia coli competent cell, and 3, carrying out inducible expression of the recombinant fusion protein by engineering bacteria obtained by the step 2, carrying out ultrasonication on the obtained engineering bacteria, then carrying out centrifugation and carrying out incubation of food-grade lactococcus lactis by the supernatant so that the fusion protein is anchored on the surface of the food-grade lactococcus lactis without protein purification. The prepared food-grade lactococcus lactis with human metallothionein-I anchored on the surface can enrich heavy metals. The preparation method has the advantages of high efficiency, safety, no toxicity, low cost and no protein purification.
Owner:SOUTH CHINA UNIV OF TECH
A method and kit for purifying protein
ActiveCN108264547BHigh removal rateImprove renaturation rateThrombopoietinPeptide/protein ingredientsBiotechnologyFermentation
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Effective renaturation liquid of human interferon inclusion body comprising D-sorbiol and renaturation method
InactiveCN1422866AImprove renaturation rateReduce manufacturing costPeptide/protein ingredientsAntiviralsInclusion bodiesGlutathione
The invention refers to the plural solution used to reform human-interferon inclusion-body protein and the plural method. The solution comprises Tris-HCL solution, EDTA, reductive-type and oxidizing-type glutathione, D-sorbierite and double-vaporing or deionized water. The method: sub-step adding sample and once plural.
Owner:武汉柏傲生物工程有限公司
A kind of refolding method of recombinant protein
InactiveCN103626834BLess volume increaseConcentration dilution is smallPeptide preparation methodsBuffer solutionProtein Renaturation
The invention relates to a recombinant protein renaturation buffer solution as well as a preparation method and an application thereof. The buffer solution consists of a basis solution, 0.5% of beta-mercaptoethanol, 1% of tween-20, 10mM of beta-cyclodextrin, 1M of L-cysteine and 4M of urea. The preparation method comprises the following steps: firstly, adding 4M of urea into the basis solution, secondly, adding 0.5% of beta-mercaptoethanol, 1% of tween-20, 10mM of beta-cyclodextrin and 1M of L-cysteine according to volume ratio, so as to form the recombinant protein renaturation buffer solution. The buffer solution provided by the invention is applied to recombinant protein renaturation with the primary protein concentration greater than 1mg / mL, is simple to operate, low in cost and convenient in industrial production, the volume after renaturation is slightly increased, the protein concentration is slightly diluted, and the renaturation rate is high.
Owner:JIANGSU ACAD OF AGRI SCI
A kind of purification production method of recombinant human bone morphogenetic protein-2
ActiveCN104447977BReduce precipitationLow costBone-inducing factorOsteogenic factorInclusion bodiesFiltration
The invention discloses a purification production method for recombinant human bone morphogenetic protein-2 (rhBMP-2 protein). The method comprises the following steps: 1) lysing an inclusion body containing rhBMP-2 protein, so as to obtain an inclusion body lysate; 2) successively performing affinity chromatography and cation exchange chromatography on the inclusion body lysate for separation purification, so as to obtain a denatured recombinant human bone morphogenetic protein-2 solution; 3) performing renaturation on the denatured rhBMP-2 protein, so as to obtain a renatured rhBMP-2 protein crude product; and 4) successively performing affinity chromatography and gel filtration chromatography for separation and purification, so as to obtain a renatured rhBMP-2 protein pure product. By using the provided method for purifying rhBMP-2 protein, the prepared product has the purity of 95% or more and the protein yield of 9.4%, and the endotoxin and DNA residues both accords with biological medicine quality requirements. The method is suitable for industrial large-scale production. Also the method provides reference for research and production of same-kind recombinant protein medicines.
Owner:YANTAI ZHENGHAI BIO TECH
A kind of renaturation method of recombinant human proinsulin fusion protein
ActiveCN105732820BSimple processing methodSimple and efficient operationAntibody mimetics/scaffoldsPeptide preparation methodsEscherichia coliHigh concentration
The invention relates to a renaturation method of restructured human insulin prokaryotic-fusion protein and belongs to the field of protein purification. The renaturation method includes steps of 1) smashing escherichia coli expressing restructured human insulin prokaryotic-fusion protein, and collecting inclusion bodies; 2) washing the inclusion bodies, dissolving the inclusion bodies and modifying the fusion protein; 3) renaturating protein. The renaturation method is needless of protein purification in advance, directly performs protein renaturation, and finally obtains high-concentration restructured insulin prokaryotic-fusion protein of natural structure. By the renaturation method, the defect of low protein concentration after protein renaturation and resultantly easy protein accumulation and precipitation is overcome, renaturation efficiency is up to 80-90%, production efficiency is improved, and the renaturation method is applicable to industrial production.
Owner:TONGHUA DONGBAO PHARMA
Renaturation separation purification method of tissue plasminogen activator mutant
InactiveCN1303207CImprove renaturation rateReduce lossesPeptide preparation methodsEnzymesEscherichia coliPurification methods
A process for renaturating, separating and purifying the mutant of tissue plasminogen activator features that the imidazole and urea are used to make the tissue plasminogen activator 'Ruitipu enzyme' be correctly folded in order to have activity and make it be renaturated, separated and purified simultaneously.
Owner:CHINA PHARM UNIV
Multi-subunit protein renaturation method
InactiveCN101343311BImprove renaturation rateIncrease productionPeptide preparation methodsComplete proteinProtein subunit
Owner:SOUTHEAST UNIV
Effective renaturation liquid of recombinant human interon inclusion body and renaturation method
InactiveCN1194015CImprove renaturation rateReduce manufacturing costPeptide/protein ingredientsAntiviralsInclusion bodiesArginine
The invention refers to the plural solution used to reform human-interferon inclusion-body protein and the plural method, belonging to bio-pharmacy field. The solution consists of 100ml 0.05-0.20mol Tris-HCL solution, pH 7.0-8.5, 0.44-0.60mol EDTA, pH 7.0-8.51, 1.2-2.55mmol reductive-type glutathione, 0.1-0.31 mmol oxidizing-type glutathione, 0.4-0.60 oml L-arginine and the remaining double-vaporing or deionized water according to the final concentration of every litre. The method: sub-step adding sample and once plural.
Owner:武汉柏傲生物工程有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com