Freeze-dried protein renaturation method
A protein refolding and protein denaturation technology, applied in the field of biopharmaceuticals, can solve problems such as excessive dilution of samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
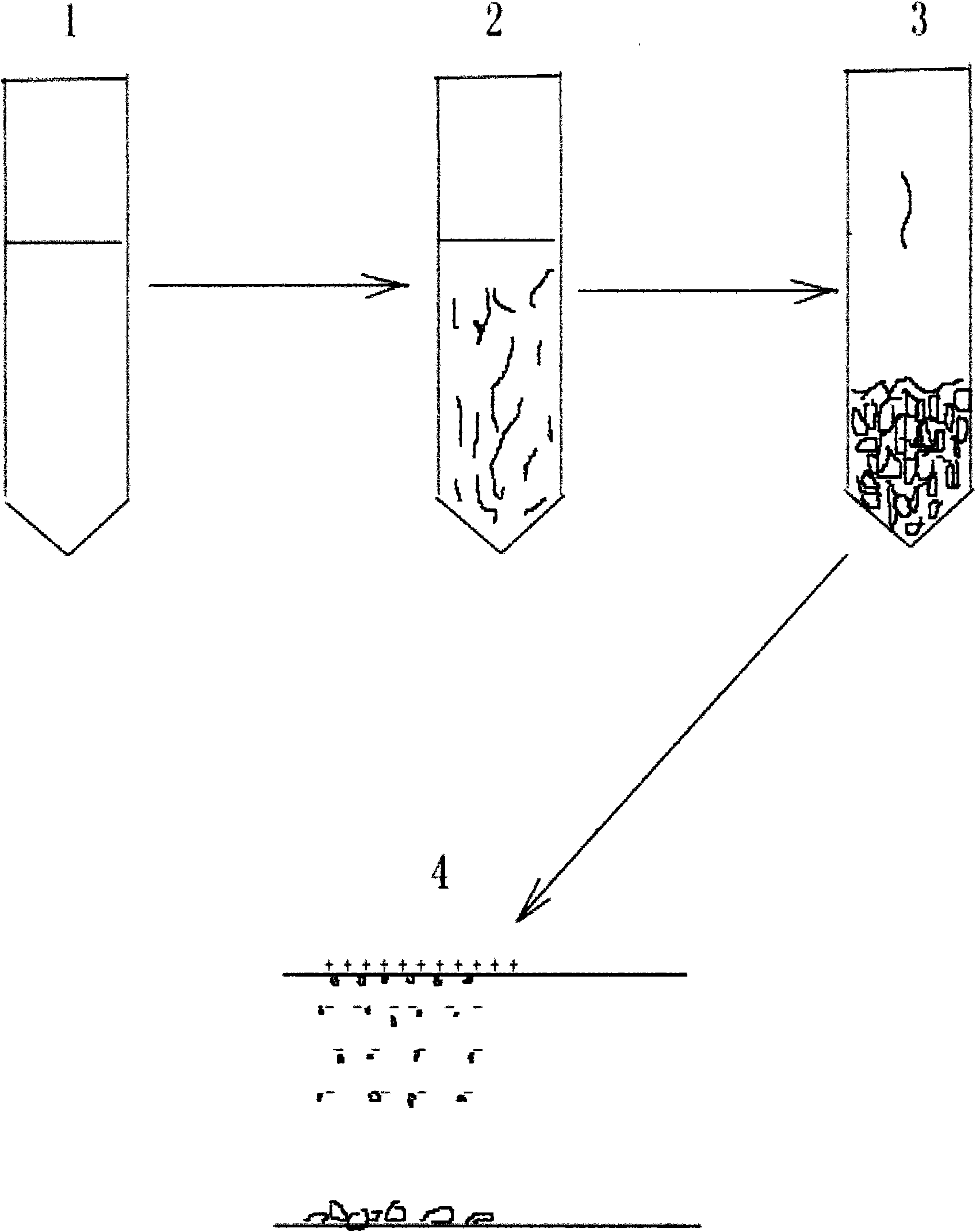
[0011] The present invention will be described in further detail in conjunction with the accompanying drawings. Step 1 Dissolve the target protein with reagents such as 6M guanidine hydrochloride or 8M urea according to the optimized biological operation formula. Step 2 needs to optimize the temperature drop rate and solution dosage for different protein pairs, and find a suitable freezing method. Step 3: sublimate water and the like in a freeze-drying vacuum dryer. Step 4 Appropriately crush the solid particle components obtained after freeze-drying, and separate the active protein powder by high-voltage electrostatic adsorption.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com