Refolding and purification method of recombinant human granulocyte stimulating factor
A technology for stimulating factors and granulocytes, applied in the field of protein purification downstream of biomedicine and bioengineering, can solve the problems of increasing the difficulty of process amplification, the difficulty of industrialized amplification, and the slow flow rate of chromatography, which is beneficial to the maintenance of protein activity, The effect of shortening the process operation time and improving the renaturation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
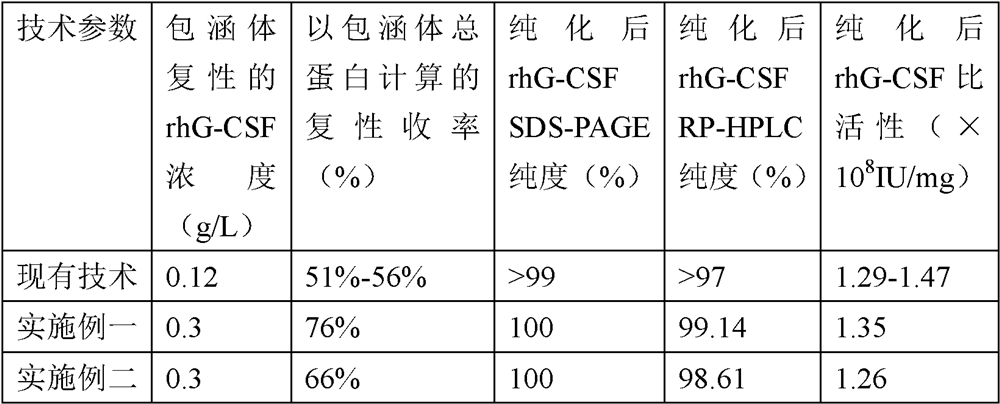
[0051] The renaturation and purification method of recombinant human granulocyte stimulating factor inclusion body mainly includes the following steps:
[0052] Step 1 Preparation of highly pure rhG-CSF inclusion bodies
[0053] 1) Process steps:
[0054] Escherichia coli DH5α strain transformed with pBV220 / G-CSF was inserted into primary seed medium (peptone 10g / L, yeast powder 5g / L, NaCl 5g / L), and cultivated at 30°C and 220rpm for 7 hours;
[0055] The primary seeds were inserted into the secondary seed medium (peptone 10g / L, yeast powder 5g / L, NaCl 5g / L, glucose 5g / L), and cultivated at 30°C and 220rpm for 17 hours;
[0056] The secondary seeds were inserted into the fermentation medium (peptone 10g / L, yeast powder 5g / L, NaCl 5g / L, glucose 5g / L, KH 2 PO 4 2.7g / L, Na 2 HPO 4 11g / L, MgSO 4 0.3g / L), cultured at 30°C until the pH and dissolved oxygen double rebound (pH 7.0, dissolved oxygen ≥ 30%), start feeding: feed medium 1 (peptone 20%, yeast powder 10%) 30-40g / ...
Embodiment 2
[0119] The renaturation and purification method of recombinant human granulocyte stimulating factor inclusion body mainly includes the following steps:
[0120] Steps 1, 2, and 3 are the same as steps 1, 2, and 3 of Embodiment 1.
[0121] Step 4 Dilution renaturation of rhG-CSF
[0122] 1) Process steps
[0123] Prepare 9.6L of buffer solution (20mmol / L Tris-HCl, pH8.2), control its temperature at 2-8°C, slowly add the denatured protein solution after replacement into the buffer solution, that is, the final protein concentration is 0.3g / L, and at the same time Add GSSG to a final concentration of 0.3mmol / L, add GSH to a final concentration of 0.1mmol / L, stir for 30 minutes, stop stirring, and stand at 2-8°C for renaturation for 16-18 hours.
[0124] 2) Sample testing:
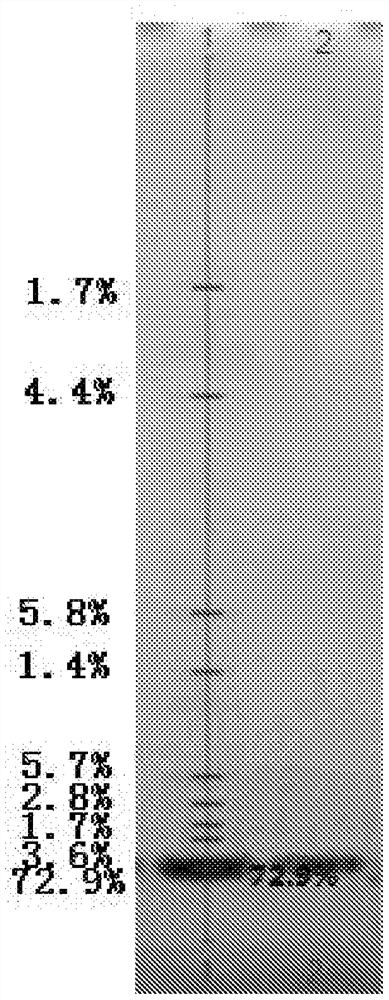
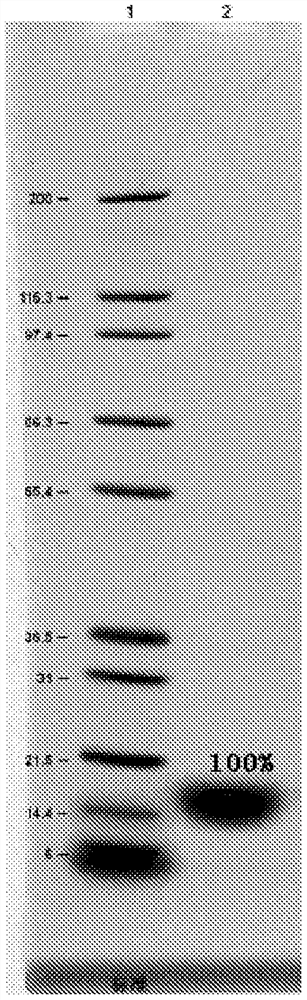
[0125] Take 50 μl of refolding solution and use RP-HPLC method to detect the rhG-CSF protein concentration in the sample (the conditions are the same as the RP-HPLC conditions in step 3), and use the area to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com