A food-grade Lactococcus lactis with surface-anchored human I-type metallothionein and its preparation method and application
A technology of Lactococcus lactis and metallothionein, which is applied in the field of preparation of food-grade Lactococcus lactis, can solve the problems of low expression level and introduction of resistance genes, etc., and achieve the effects of reducing protein loss, low cost, and omitting cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Construction of Recombinant cA-hMT Protein Engineering Bacteria
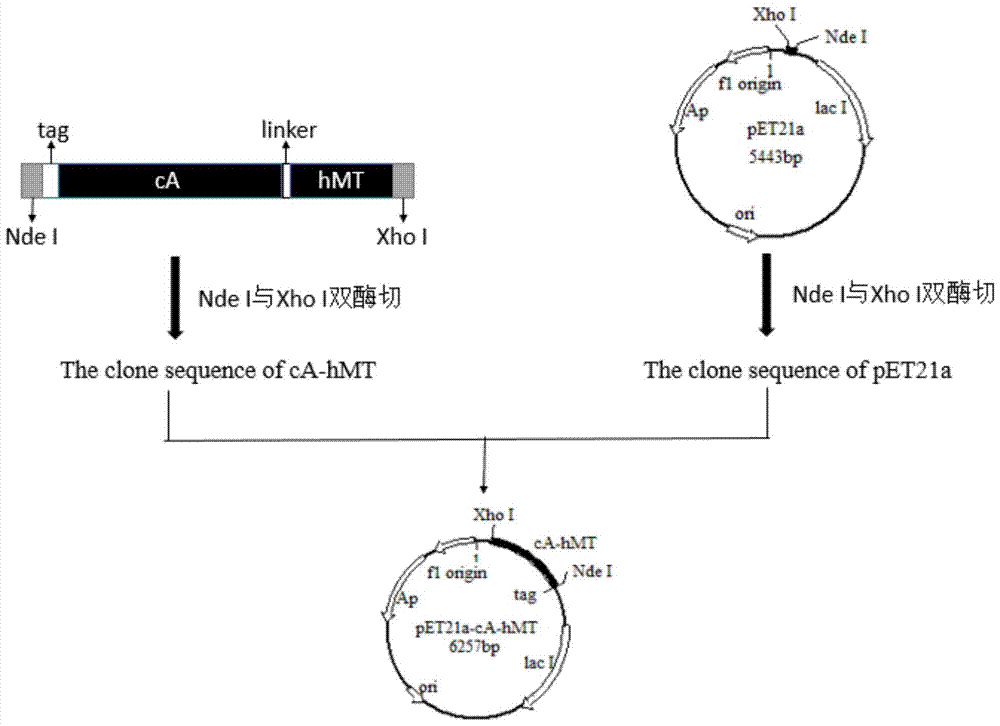
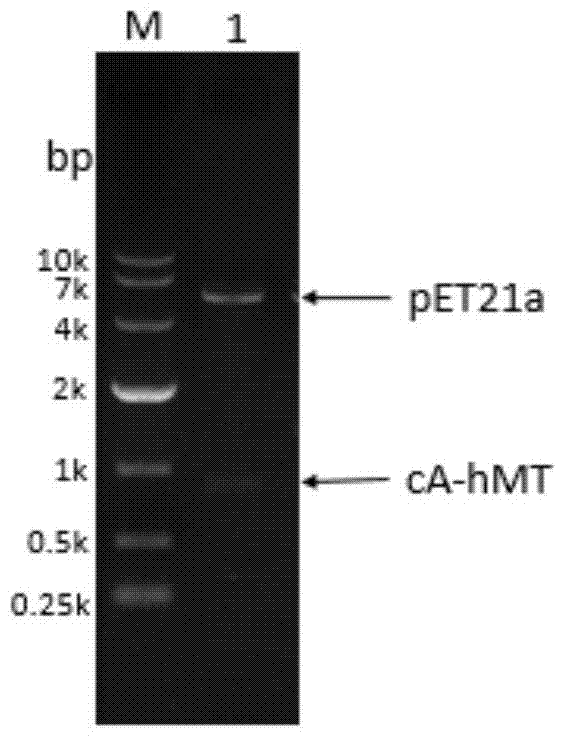
[0045] The cA gene and the hMT gene were spliced by overlapping PCR, a linker was introduced between the two genes, and an expression-promoting tag was introduced at the N-terminus. After double digestion with NdeI and XhoI, it was ligated with the expression vector pET21a to realize the recombinant plasmid pET21a-cA-hMT The construction of , the specific construction process is as follows figure 1 shown.
[0046] 1. Design and synthesis of target gene fragments and primers
[0047] According to the Lactococcus lactis MG1363 N-acetylmuramidase gene sequence (GenBank ID: CAL96887.1), a linker (SEQ ID No.3) was introduced at its C-terminus, thereby designing primers, wherein the forward primer is F1:
[0048] 5'-AATTC CATATG GGATCCAATACTAATTCTGGTGGCTC-3', containing restriction site NdeI (underlined part), the reverse primer is R1:
[0049] 5'-TGAACAATTAGGATCCATCTGCAGACCACCACCTTTTATTCGTAGATACT-3', co...
Embodiment 2
[0079] Expression and Identification of cA-hMT Recombinant Protein
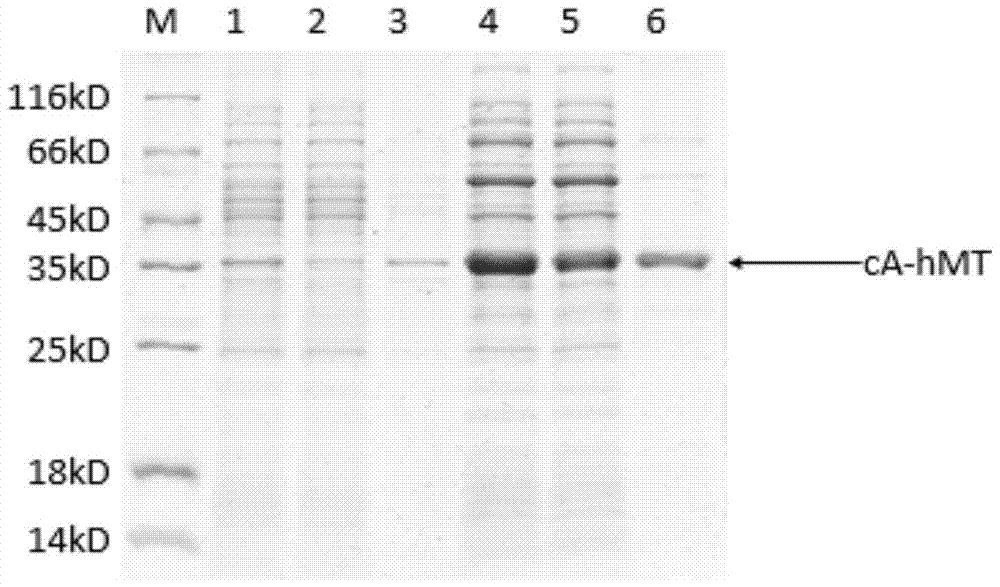
[0080] Method: The plasmids extracted from the positive clones in the above-mentioned Example 1 and the plasmids containing molecular chaperones (dnaK, dnaJ, grpE, groES and groEL) were transformed into Escherichia coli expression strain competent cells BL21 (DE3), picked and identified by colony PCR The positive clones were inoculated into 3 mL of LB liquid medium containing 100 μg / mL Amp, 25 μg / mL chloramphenicol, and 1 mg / mL L-arabinose for expansion at 37 ° C. 600 Add 0.5mM IPTG when it is 0.5, collect the bacteria by centrifugation after 12 hours of low-temperature induction, ultrasonically lyse, add loading buffer to the supernatant and precipitate, and boil in water for 5 minutes. Concentrating gel 80V, separating gel 120V for SDS-PAGE electrophoresis, after electrophoresis, stain with Coomassie brilliant blue staining solution, and decolorize with decolorizing solution.
[0081] Result: if image 3 ...
Embodiment 3
[0084] Anchoring of recombinant hMT fusion protein to lactic acid bacteria and detection
[0085] 1. Culture of Lactococcus lactis and preparation of acid-treated Lactococcus lactis
[0086] method:
[0087]Streak a small amount of glycerol-frozen strains onto the solid medium of GM17 plates, and place them in an incubator at 30°C for static culture for 24 hours. Pick a single colony and inoculate it in 5 mL of GM17 liquid medium and culture it statically at 30°C for 12 hours. Take the overnight culture of L. lactis MG1363, inoculate it into 500 mL of fresh GM17 medium at a ratio of 1:100, and culture it statically at 30°C for 16 hours. Collect the cells by centrifugation, wash once with 250 mL of sterile water, resuspend the cells in 100 mL of acetic acid solution (pH=1), and boil in boiling water for 30 min. Wash 3 times with 250 mL of sterile PBS buffer, resuspend the bacteria with 50 mL of sterile PBS buffer, and store at 4°C.
[0088] 2. Back anchoring of cA-hMT prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com