A kind of purification production method of recombinant human bone morphogenetic protein-2
A morphogenetic protein separation and purification technology, applied in the field of protein purification, can solve the problems of unsuitability for industrial scale production, easy precipitation of protein, low yield, low renaturation rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, purification of recombinant human bone morphogenetic protein-2
[0059] 1. Dissolution of inclusion bodies
[0060] Inclusion body: the inclusion body containing the recombinant human bone morphogenetic protein-2 (rhBMP-2, sequence 1) was obtained through prokaryotic expression of Escherichia coli disclosed in the patent CN200510132792.9.
[0061] Inclusion body solution: 0.1mol / L Tris-HCl, 8mol / L guanidine hydrochloride, 10mmol / L DDT, pH9.0. Each concentration is the final concentration of the corresponding component in the solution.
[0062] Inclusion bodies were dissolved at a ratio of 1 gram of inclusion body: 30 mL of inclusion body lysate, shaken at 4°C and 150 r / min for 18 hours, centrifuged at 10,000 g for 10 min at high speed, and the supernatant was collected as inclusion body lysate.
[0063] 2. Purification before renaturation
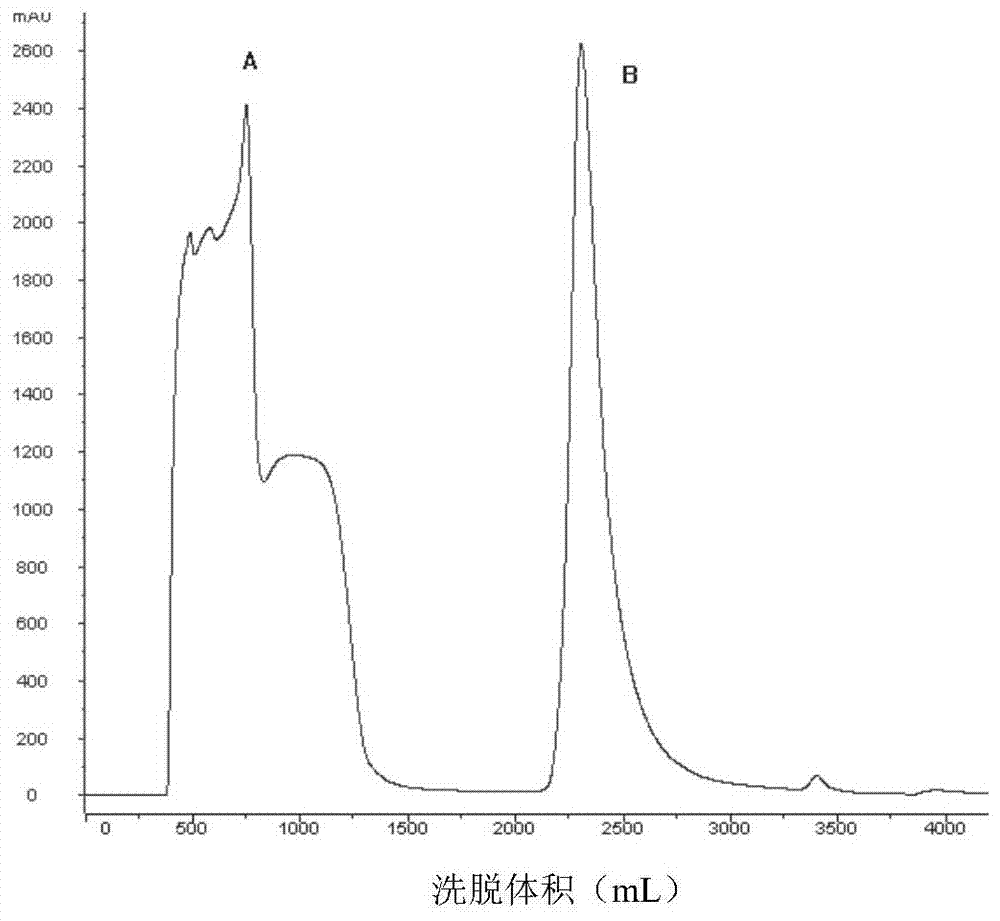
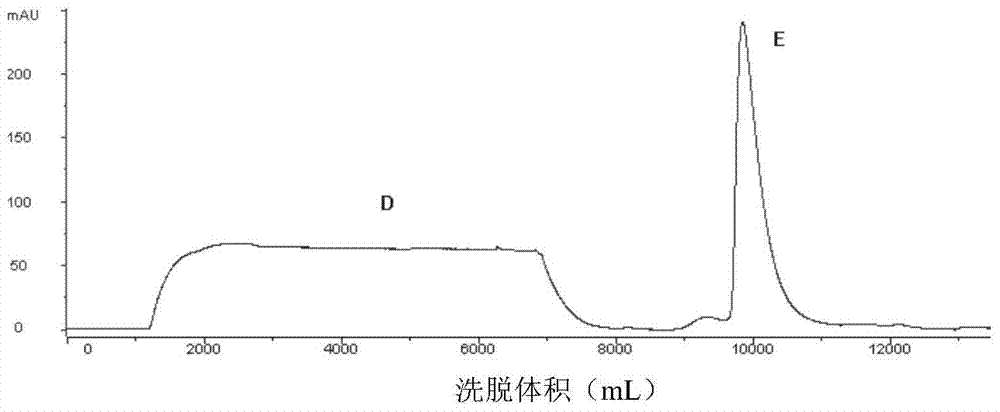
[0064] Affinity chromatography and cation exchange chromatography were performed sequentially before protein renat...
Embodiment 2
[0109] The quality detection of embodiment 2, embodiment 1 gained protein product
[0110] 1. Detection of protein purity
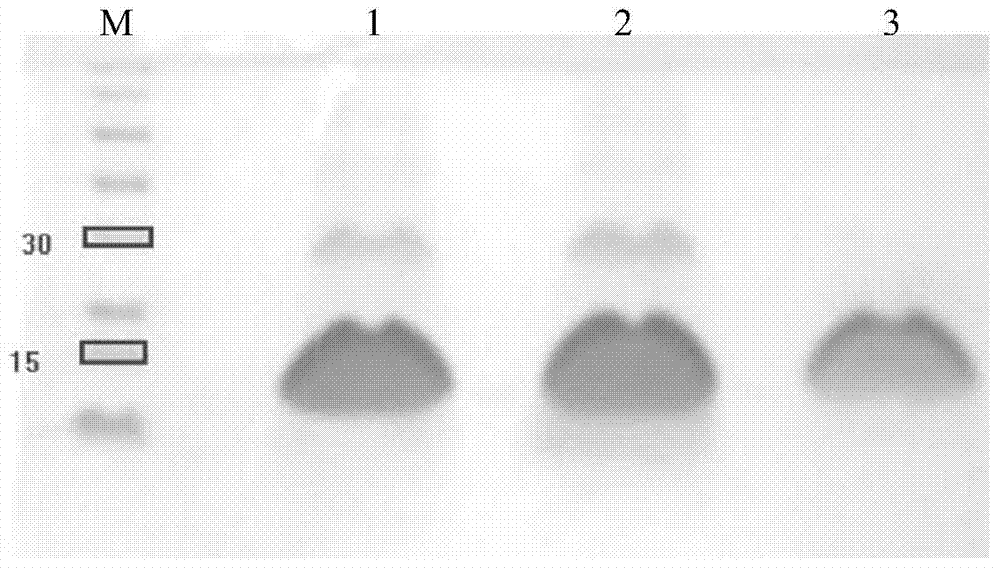
[0111] Referring to Appendix IVC of the third part of the "Chinese Pharmacopoeia" in 2010, the purity of the protein stock solution obtained in Example 1 was tested by non-reducing SDS-polyacrylamide gel electrophoresis. The concentration of the separating gel was 15%, and the sample volume was not less than 10 μg (Coomassie Brilliant Blue R250 staining method).
[0112] The results showed that the purity of the rhBMP-2 dimer in the elution peak finally collected in Example 1 was 98.3% after scanning by the scanner. Such as Figure 7 shown.
[0113] 2. Calculation of protein yield
[0114] Referring to Appendix VIB of the third part of the "Chinese Pharmacopoeia" in 2010, the protein concentration of the protein stock solution obtained in Example 1 was measured by the lowrry method to be 0.75 mg / mL, and the rhBMP-2 stock solution could be obtained by pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com