A cyclic chromatography renaturation method for recombinant human bone morphogenetic protein-2
A morphogenetic protein and circulating layer technology, which is applied in the fields of bone morphogenetic factors, peptide preparation, animal/human proteins, etc. problems, to achieve the effect of being suitable for industrial scale production, reducing protein precipitation, and small processing volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
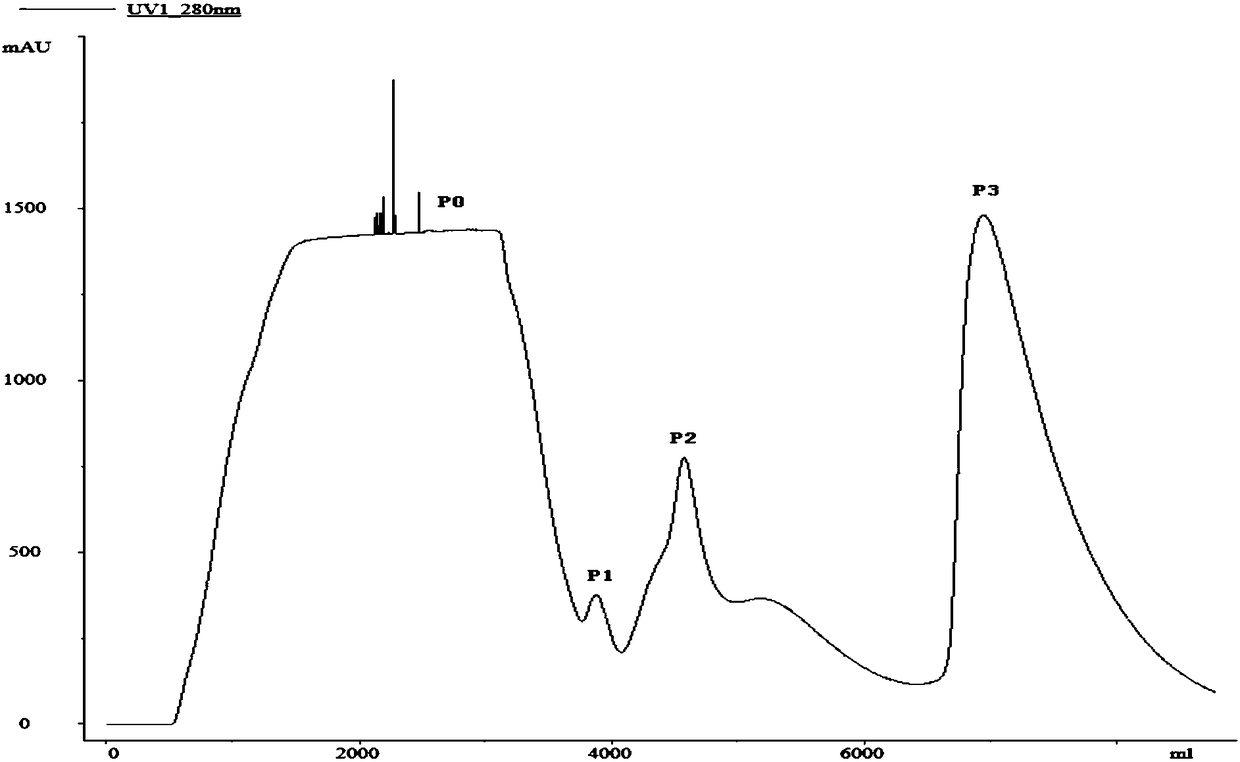
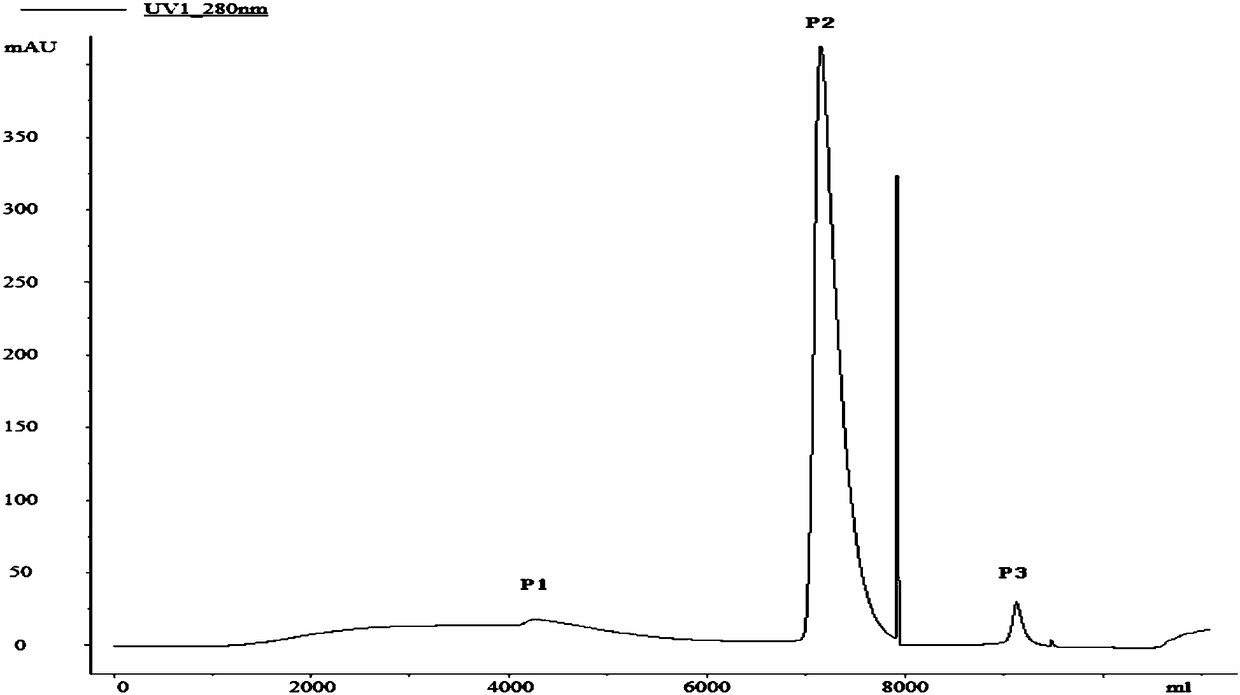
[0074] Example 1. Refolding of recombinant human bone morphogenetic protein-2rhBMP-2 inclusion bodies by affinity chromatography
[0075] 1. Denaturation of rhBMP-2 inclusion bodies
[0076] Inclusion body solution: 0.1mol / L Tris-HCl, 8mol / L urea, 0.1mol / L DTT, 5mmol / L EDTA; pH8.5. Each concentration is the final concentration of the corresponding component in the solution, and the solvent is water.
[0077] Denaturation: Dissolve inclusion bodies at a ratio of 1 gram of recombinant human bone morphogenetic protein-2rhBMP-2 inclusion body: 10 mL of inclusion body solution, shake at 25°C and 100 r / min for 6 hours, centrifuge at 12,000 g for 30 minutes at high speed, and collect the supernatant The solution is denatured rhBMP-2 protein solution.
[0078] 2. Refolding of rhBMP-2 by affinity chromatography after denaturation
[0079] 1. Pre-purification
[0080] In order to improve the purity of the protein and reduce the content of impurities such as nucleic acid, the denatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com