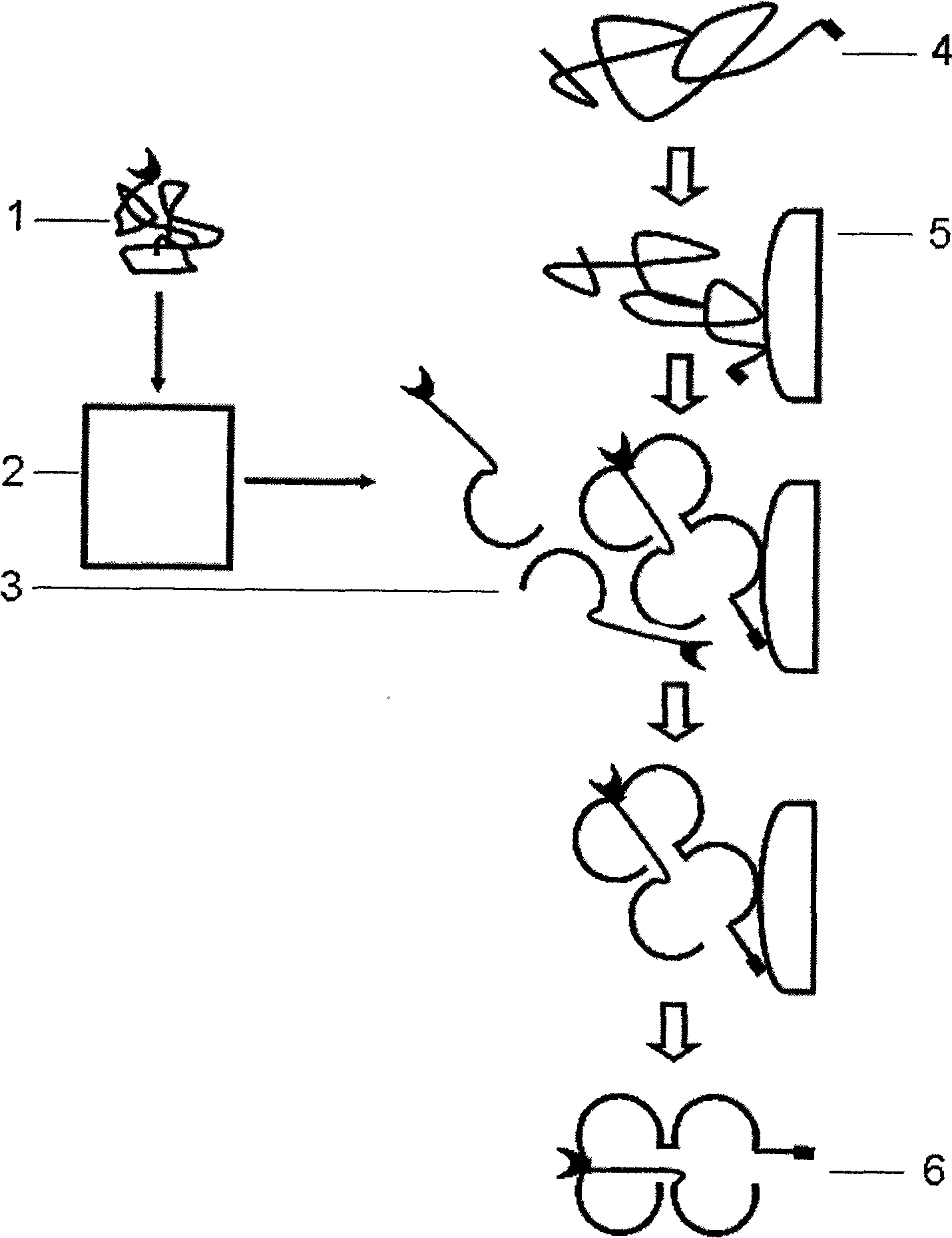
Multi-subunit protein renaturation method
A multi-subunit protein and protein technology, applied in the fields of biochemistry and molecular biology, can solve the problem of no chromatographic refolding method, and achieve the effect of saving raw materials and simplifying the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Amplification of MHCI heavy chain gene and construction of recombinant plasmid.
[0031]Primers were designed according to the cDNA sequence of human MHC I heavy chain in GenBank (AF036921.1GI: 4104536). At the C-terminus of the heavy chain gene, there is a BSP (biotin protein ligaseBirA substrate peptide) sequence (17aa: GSLHHILDAQKMVWNHR) for biotinylation and can be combined with Ni 2+ - NTA-bound His-tag (6aa: HHHHHH). The upstream primer contains start codon, NcoI recognition site and protection base, and the downstream primer contains XhoI restriction site. Their sequences are:
[0032] Upstream p1: 5`-GCCCATGGGCTCTCACTCCATGAGGTAT-3`;
[0033] Downstream p2: 5`-GCCTCGAGACGATGATTCCACACCATTTTCTGTGCATCCAGAATATGATGCAGGGATCCGGTGAGGGGCTTGGGCAA-3`.
Embodiment 2
[0036] Example 2: Extraction of human peripheral blood cell total RNA and HBc 18-27 - Construction of recombinant plasmids for β2 microglobulin.
[0037] (1) Amplification of β2 microglobulin (β2m) cDNA
[0038] The extraction steps of total RNA from human peripheral blood cells were carried out according to the method recommended by the TRIZOL kit (Promega).
[0039] Primers were designed according to the cDNA sequence of human β2m in GenBank (S71244.1GI:547297).
[0040] The upstream primer contains start codon, NcoI recognition site and protection base, and the downstream primer contains XhoI restriction site. Their sequences are:
[0041] Upstream p3: 5`-GGCCCATGGATATCCAGCGTACTCCAAAG-3`;
[0042] Downstream p4: 5`-CAACTCGAGattaCATGTCTCGATCCCACTTAAC-3`.
[0043] The reverse transcription polymerase chain reaction "RT-PCR" system and process used to amplify the β2 microglobulin gene are as follows: add 6 μl DEPC water to the 20 μl total reaction system, add about 5 μl o...
Embodiment 3
[0054] Example 3: MHCI heavy chain protein and HBc 18-27 -Expression and preliminary purification of β2 microglobulin
[0055] MHCI heavy chain and HBc 18-27 -β2 microglobulin recombinant plasmids were transformed into host bacteria E.coli BL21(DE3), picked clones and inoculated in LB medium containing kanamycin (35μg / ml), cultured to OD 600 0.6-0.8, add IPTG to a final concentration of 0.1mM, and induce at 37°C for 3h. Collect the bacterial pellet by centrifugation at 5000g for 10min, and resuspend in ice-cold Buffer A (20mM Tris, 150mM NaCl, pH8.0), mix well and perform high-pressure homogeneous destruction, centrifuge at 10000g at 4°C for 10min, discard the supernatant, and weigh the precipitate Suspend in Buffer B (20mM Tris, 150mM NaCl, 0.5% Triton X-100, pH8.0), mix well and centrifuge at 10000g at 4°C for 10min, discard the supernatant, and collect the inclusion body precipitate. Then repeat the wash once, resuspend the pellet in Buffer B, mix well and centrifuge. R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com