A kind of renaturation method of recombinant human proinsulin fusion protein
A technology of recombinant human insulin and fusion protein, which is applied in the field of protein renaturation, can solve the problems of low renaturation rate, complex production process, and low protein concentration, and achieve the effect of improving renaturation rate, simple processing method and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
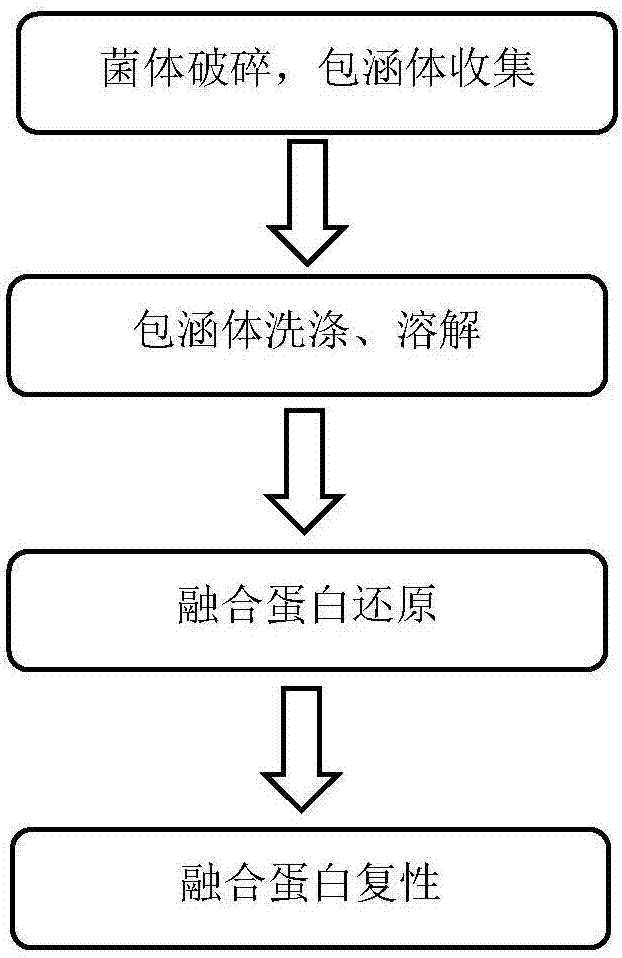
[0040] Preparation of recombinant human proinsulin fusion protein
[0041] Step 1: Collection of recombinant human proinsulin fusion protein inclusion bodies
[0042] After fermentation by Escherichia coli, wash twice with purified water, determine the weight, add 100mL of purified water for every 10g of bacteria, mix well, crush in a homogenizer at 900-950bar, after the bacteria are completely broken, use a centrifuge Centrifuge at 12 000g for 10 minutes to collect the precipitate. The obtained precipitate is mainly inclusion bodies. Wash twice according to the following method to remove most of the non-recombinant human proinsulin fusion protein: use 100mL washing buffer for every 10g of precipitate, containing 50mM Tris-HCl (pH9.0), 2mM EDTA, 1% Triton-X100 (mass ratio), 3M urea. Stir and mix at room temperature, let stand for 30 minutes, and centrifuge at 12 000 g for 10 minutes, so as to collect the precipitate twice.
[0043]Step 2: Dissolution and reduction of recombi...
Embodiment 2
[0048] Preparation of recombinant human proinsulin fusion protein
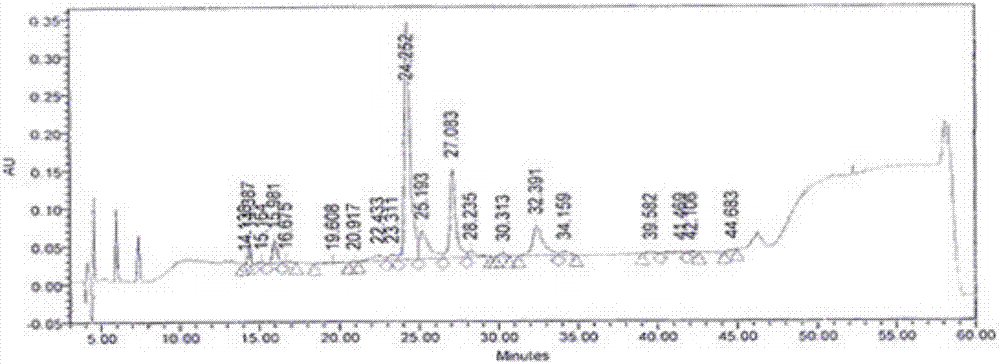
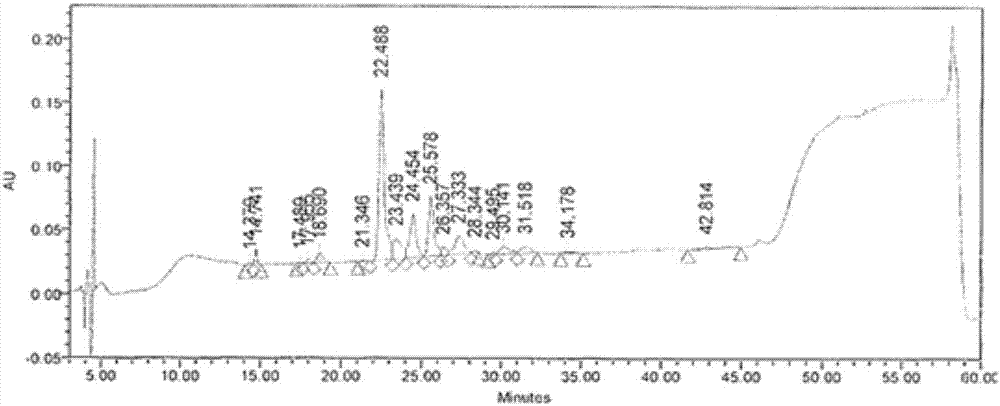
[0049] The recombinant human proinsulin fusion protein after denaturation and reduction, in the refolding buffer, the protein concentration reaches 7.5mg / mL, and the concentration of other components is: urea with a final concentration of 0.8M, oxidized glutathione with a final concentration of 1mM Glycine, reduced glutathione with a final concentration of 1mM, EDTA with a final concentration of 2mM, and β-mercaptoethanol with a final concentration of 2mM were adjusted to pH 9.0-9.5, and placed at 4°C. It can be observed that in the refolding solution, the solution remains transparent and no precipitation occurs. Other operations are the same as in Example 1. The calculated renaturation rate of recombinant human proinsulin fusion protein was 85.34%.
Embodiment 3
[0051] Preparation of recombinant human proinsulin fusion protein
[0052] The recombinant human proinsulin fusion protein after denaturation and reduction, in the refolding buffer, the protein concentration reaches 10mg / mL, and the concentration of other components is: urea with a final concentration of 0.8M, and oxidized glutathione with a final concentration of 5mM Peptide, reduced glutathione at a final concentration of 1 mM, EDTA at a final concentration of 2 mM, and β-mercaptoethanol at a final concentration of 3 mM, adjusted to pH 9.0-9.5, and placed at 4°C. It can be observed that in the refolding solution, the solution remains transparent and no precipitation occurs. Other operations are the same as in Example 1. The calculated refolding rate of recombinant human proinsulin fusion protein was 82.59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com