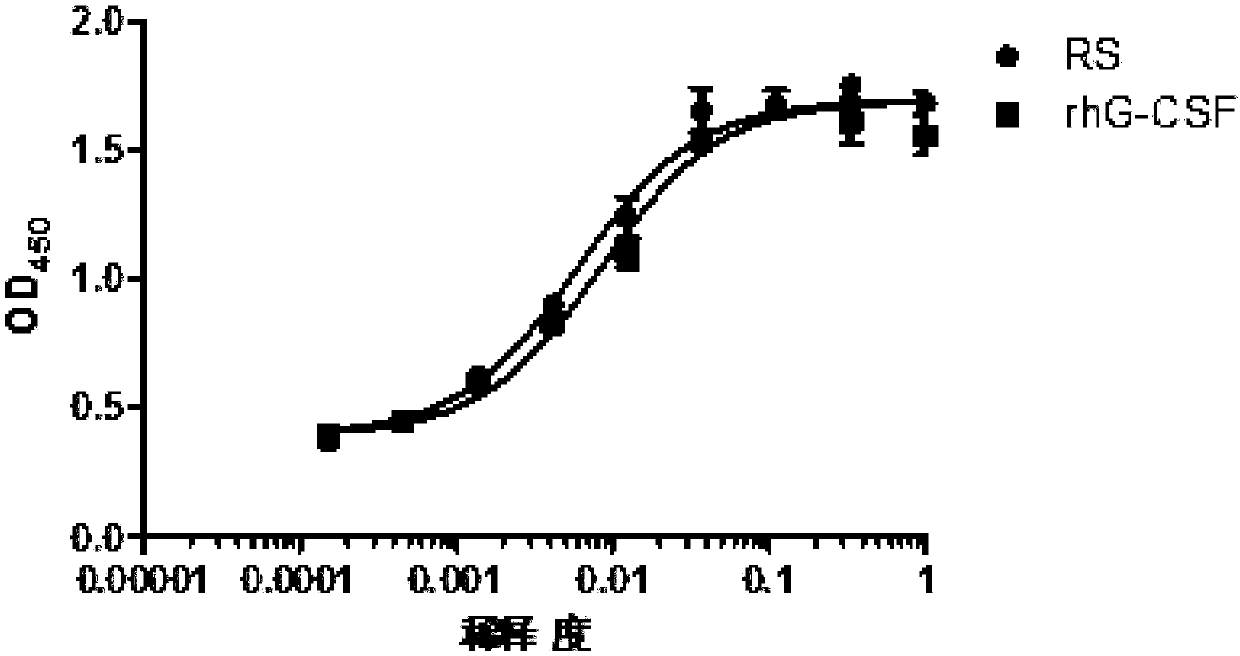
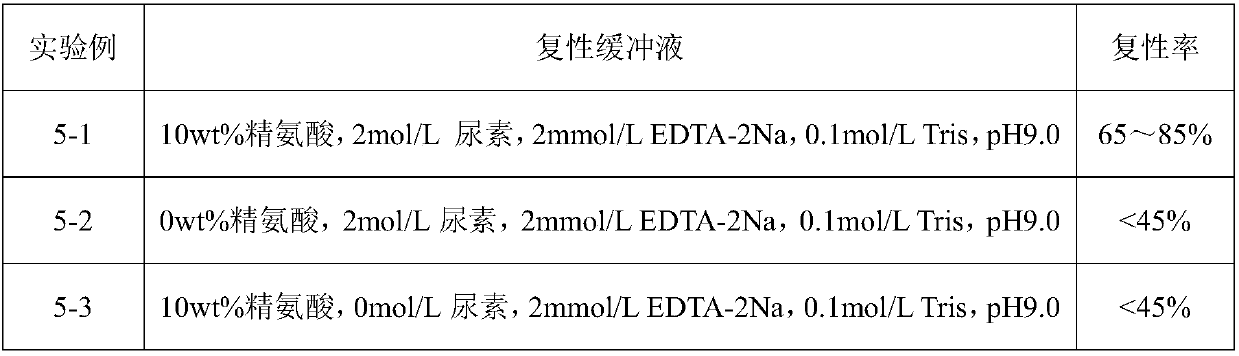
Renaturation and purification method of recombinant human granulocyte colony stimulating factor
A colony-stimulating factor and purification method technology, which is applied in the downstream protein purification field of biomedicine and bioengineering, can solve the problems of complex operation, low renaturation rate, and long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Construction of pET-9a-G-CSF prokaryotic expression strain
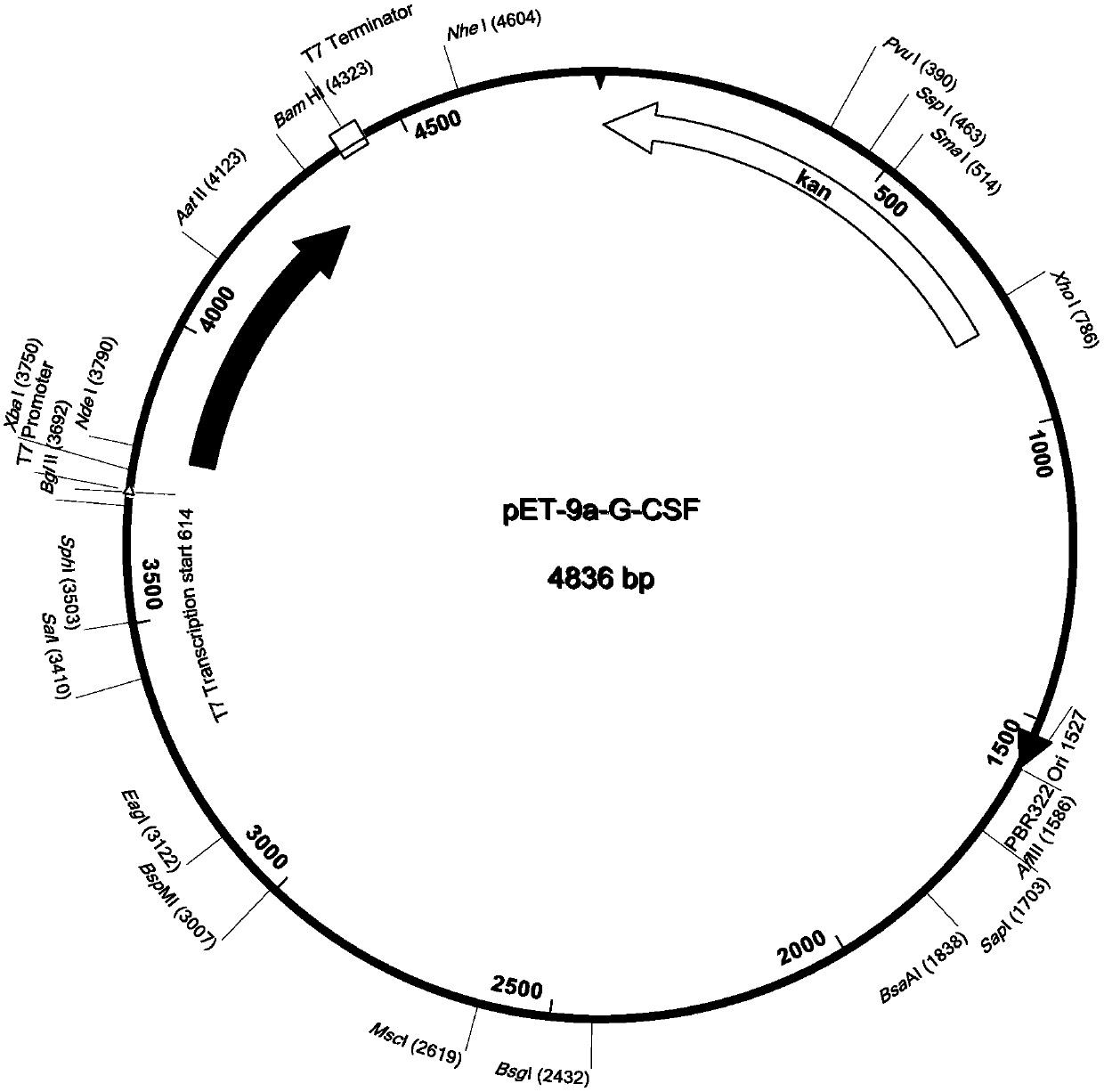
[0069] The human G-CSF gene sequence is shown in SEQ ID No.1. Synthesize the human G-CSF gene whose sequence is shown in SEQ ID No.1 according to conventional molecular cloning methods and introduce the terminator TAA and the NdeI and BamHI restriction sites at both ends of the target gene; use NdeI and BamHI to prokaryotic expression plasmid vector pET-9a and the human G-CSF gene fragment were double-digested respectively, and the double-digested pET-9a and the human G-CSF gene fragment were connected to obtain the pET-9a-G-CSF recombinant plasmid (such as figure 1 ) into the E.coli DH5α strain.
[0070] Inoculate the E.coli DH5α strain containing the pET-9a-G-CSF recombinant plasmid into LB medium containing 50 μg / mL kanamycin at an inoculum size of 1%, and cultivate overnight at 37°C on a shaker (180rpm) , using a plasmid-free endotoxin extraction kit (purchased from Biomega) to extract and pur...
Embodiment 2
[0072] 1. Preparation of rhG-CSF refolding solution
[0073] The strain used in the process is the Escherichia coli DH5α strain transformed with the pET9a / G-CSF plasmid in Example 1. After fermentation, the bacterial cells are collected, the cells are crushed by a high-pressure homogenizer, and the crude inclusion bodies are obtained by centrifugation, and then the crude inclusion bodies are washed and centrifuged to obtain preliminary purified inclusion bodies.
[0074] Take 60 g of initially purified inclusion bodies, denature and dissolve them with inclusion body lysate (6 mol / L guanidine hydrochloride, 0.15 mol / L Tris, pH 8.0) at a solid-to-liquid ratio of 1:10 (g / mL), and stir at room temperature Add DTT (dithiothreitol) to a final concentration of 7 mmol / L until it becomes clear and transparent, continue stirring for more than 30 minutes, and filter through a 0.22 μm filter to obtain a solution of inclusion bodies.
[0075] Slowly pump the inclusion body solution into 1...
Embodiment 3
[0095] 1. Preparation of rhG-CSF refolding solution
[0096] The bacterial strain used in the process is Escherichia coli DH5α strain transformed with pET9a / G-CSF plasmid. After fermentation, the bacterial cells are collected, the cells are crushed by a high-pressure homogenizer, and the crude inclusion bodies are obtained by centrifugation, and then the crude inclusion bodies are washed and centrifuged to obtain preliminary purified inclusion bodies.
[0097] Take 60 g of initially purified inclusion bodies, denature and dissolve them with inclusion body lysate (5 mol / L guanidine hydrochloride, 0.1 mol / L Tris, pH 9.0) at a solid-to-liquid ratio of 1:10 (g / mL), and stir at room temperature Add DTT (dithiothreitol) to a final concentration of 10mmol / L until it becomes clear and transparent, and continue to stir for more than 30 minutes. After filtering through a 0.22μm filter, the inclusion body solution is obtained.
[0098] Slowly pump the inclusion body solution into 10L of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com