P2Y6 receptor agonists for treating lung diseases
A lung, disease technology, applied in the field of P2Y6 receptor agonist compounds, can solve problems such as lack of understanding and low potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
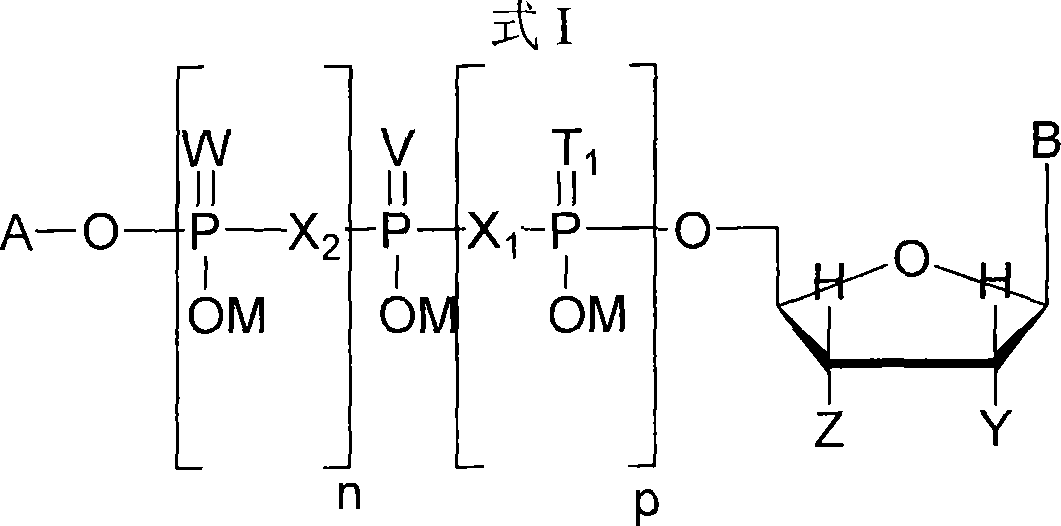
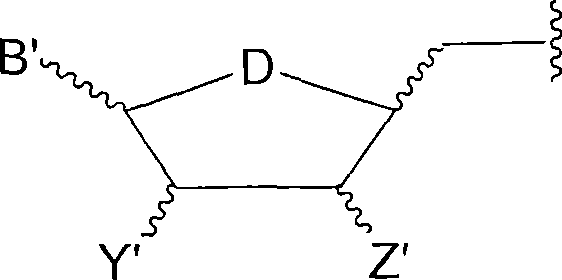
[0128] Example 1. Preparation of P 1 -(2',3'-Benzyluridine 5'-)P 3 -(Uridine 5'-)ammonium triphosphate
[0129] Uridine 5'-tributylammonium diphosphate (UDP.NBu 3 )
[0130] Dowex 50 H with uridine 5'-diphosphate disodium (UDP.2Na) and water + Or equivalent (5g resin / gUDP.2Na) was stirred for 10 minutes to convert it to the free acid. The resin was filtered and the filtrate was mixed with tributylamine (1.5 equiv). The mixture was stirred vigorously for 15 minutes to keep the pH of the aqueous layer above 8. The solution was evaporated below 35°C and the residue was co-evaporated with anhydrous N,N-dimethylformamide (3X) below 40°C. The residue was lyophilized overnight to give a dry glassy foam.
[0131] Uridine 5'-tributylammonium monophosphate (UMP.NBu 3 )
[0132] The free acid form of uridine 5'-monophosphate (UMP) was converted to the mono-tributylammonium salt by treatment with aqueous tributylamine (1.5 equivalents). After removal of the solvent and co-evapora...
Embodiment 2
[0138] Embodiment 2. UDP, UP 3 U and monobenzyl acetal UP 3 U's selectivity
[0139] In 96-well plates will express P2Y 1 、P2Y 2 、P2Y 4 and P2Y 6 Human astrocytoma (1321N1) cells were cultured to confluence. Add the Fluo-3AM (2.5 μM final concentration) solution prepared with assay buffer consisting of 10 mM KCl, 118 mM NaCl, 2.5 mM CaCl 2 , 1mM MgCl 2 , 20mM HEPES, 10mM glucose, pH7.4 composition. After incubating with Fluo-3 AM at 25°C for 60 minutes, the cells were washed and treated with serially diluted concentrations of the compounds uridine 5'-diphosphate (UDP), P 1 , P 3 -(Diuridine 5'-)triphosphate (UP 3 U) or P 1 -(2',3'-Benzyluridine 5'-)P 3 -(Uridine 5'-)triphosphate (monobenzyl acetal UP 3 U) stimulation. Changes in fluorescence density were measured using FLIPR (Molecular Devices Corp., Sunnyvale, CA) while intracellular calcium levels were monitored in each well. The results of this experiment are shown in Table 1. Compound UDP, UP in Table 1 3 ...
Embodiment 3
[0144] Example 3. Chloride secretion by human nasal airway cells.
[0145] Induction of chlorine secretion in vivo can help to hydrate thick airway mucus secretions in patients with disease, allowing patients to benefit from the mobilization and clearance of these secretions. Activation of apical non-CFTR chloride channels induces chloride ion and water outflow, thereby aiding rehydration of pulmonary secretions (Boucher, US Patent 5,292,498 and Boucher, US Patent 5,635,160, and references cited therein).
[0146] Airway epithelial cells were dispersed and isolated from freshly excised human nasal surgical specimens (Yankaskas et al., Am Rev Resp Dis 132, 1281-1287 (1985)). Confluent monolayers of cells were grown on permeable collagen matrix supports in F-12 hormone-supplemented medium (Wu, et al., Am Rev Resp Dis 132, 311-320 (1985)). Cells were incubated at 37°C and grown to confluence. The occurrence of transepithelial electrical resistance was monitored to determine the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com