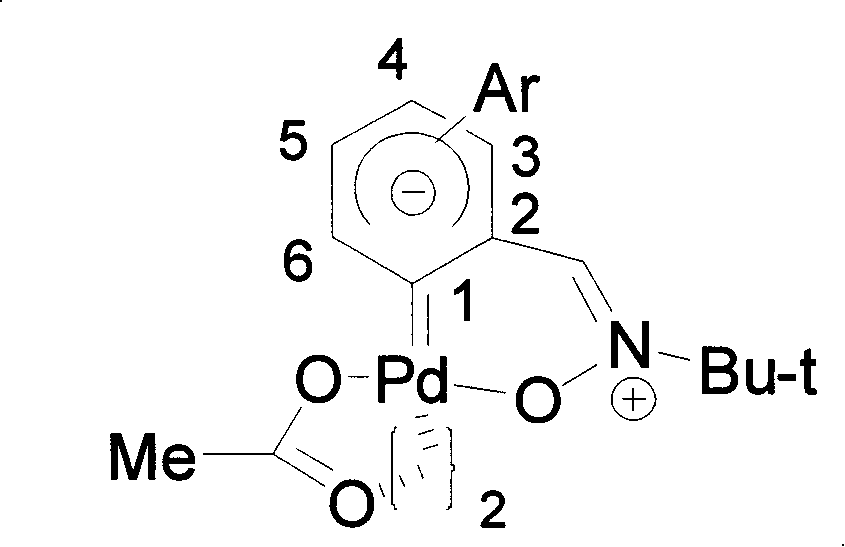
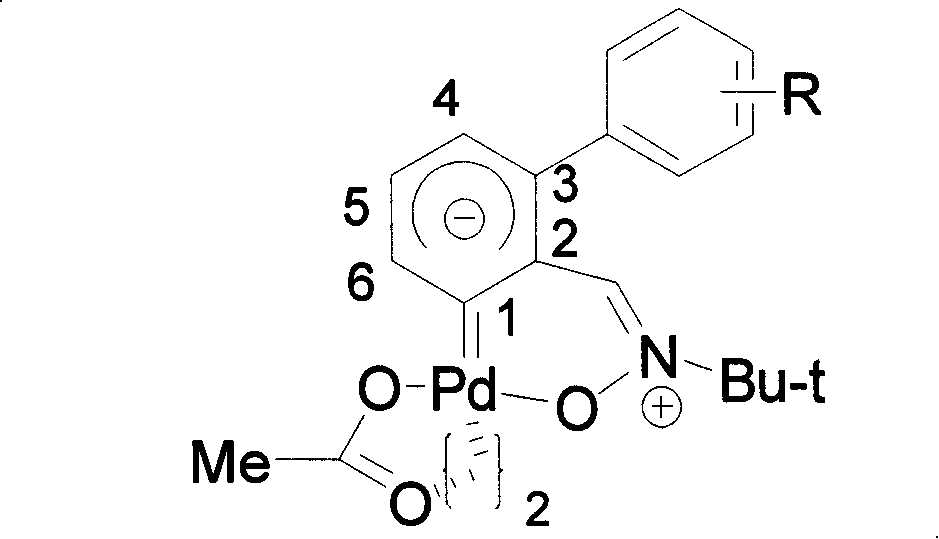
Biphenyl nitrone carbocyclic carbene-palladium complex and method for synthesizing same
A technology of biphenyl nitrone and a synthesis method is applied in the field of α-biphenyl nitrone carbocyclic carbene-palladium complex and its synthesis, and can solve the problems of difficulty in purifying reaction operation products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve α-[2-(4-methoxyphenyl)]phenyl-N-tert-butylnitrone (142 mg, 0.5 mmol) and palladium diacetate (113 mg, 0.5 mmol) in glacial acetic acid (2mL ), the reaction was refluxed for 10 hours. After the reaction, the solvent was removed under reduced pressure, and then separated and purified by silica gel column chromatography to obtain the α-biphenone carbene-palladium complex of structural formula (III).
[0040]
[0041] Formula (III)
[0042] In the formula, R is p-methoxy.
[0043] When silica gel column chromatography is used for separation and purification, first eluted with ethyl acetate / petroleum ether = 1 / 4 to remove impurities, and then eluted the product with ethanol.
[0044] The yield of the α-biphenylnitrocarbene-palladium complex was 74%. Mp: 176-178°C; a mixture of trans and cis isomers (trans / cis=77 / 23); 1 H NMR(400MHz, CDCl 3 ): Anti-isomer (major): δ7.68(s, 2H), 7.47(d, J=7.6Hz, 2H), 7.05-7.77(m, 12H), 3.87(s, 6H), 2.09( s, 6H), 1.02 (s, 18H); cis-isome...
Embodiment 2
[0046] The synthesis method is the same as in Example 1, except that α-[3-(4-methoxyphenyl)]phenyl-N-tert-butylnitrone is used instead of α-[2-(4-methoxyphenyl) ]Phenyl-N-tert-butylnitrone. Obtain the α-bifenone carbene-palladium complex of structural formula (IV),
[0047]
[0048] Formula (IV)
[0049] In the formula, R is p-methoxy.
[0050] The yield of the α-biphenylnitrocarbene-palladium complex was 56%. Mp: 170-172°C; a mixture of trans and cis isomers (trans / cis=91 / 9); 1H NMR(400MHz, CDCl 3 ): Anti-isomer (major): δ 7.53 (d, J = 8.4 Hz, 2H), 7.51 (s, 2H), 7.43 (d, J = 8.4 Hz, 4H), 7.27 (d, J = 8.4Hz, 2H), 7.11 (s, 2H), 6.95 (d, J=8.4Hz, 4H), 3.84 (s, 6H), 2.12 (s, 6H), 1.14 (s, 18H); cis-isomer Body (minor, selected peak): 1.51 (s, 18H); 13 C NMR(100MHz, CDCl 3 ): Anti-isomer δ180.8, 158.9, 138.1, 136.7(x2), 134.8, 132.8, 127.5(x2), 126.8(x2), 125.3, 114.2(x 2), 69.5, 55.3, 27.9(x 3 ), 24.3. cis-isomer (minor, selected peaks) 127.7(×2), 114.1(×2), 28.3(×3).
Embodiment 3
[0052] The synthesis method is the same as in Example 1, the difference is that α-[4-(4-methoxyphenyl)]phenyl-N-tert-butylnitrone is used instead of α-[2-(4-methoxyphenyl) ] Phenyl-N-tert-butyl nitrone was refluxed in 5 mL of glacial acetic acid for 5 hours to obtain the α-biphenyl nitro carbene-palladium complex of structural formula (V),
[0053]
[0054] Formula (V)
[0055] In the formula, R is p-methoxy.
[0056] The yield is 60%. Mp: 174-176°C; a mixture of trans and cis isomers (trans / cis=90 / 10);
[0057] 1 H NMR(400MHz, CDCl 3 ): Anti-isomer (major): δ7.71(s, 2H), 7.54(d, J=8.4Hz, 4H), 7.46(s, 2H), 7.22(d, J=7.6Hz, 2H) , 7.01 (d, J = 7.6 Hz, 2H), 6.96 (d, J = 8.4 Hz, 4H), 3.85 (s, 6H), 2.12 (s, 6H), 1.05 (s, 18H); cis-isomer Body (minor, selected peaks): 1.49 (s, 18H); 13 C NMR(100MHz, CDCl 3 ): Anti-isomer (major): δ180.7, 159.3, 139.7, 138.4, 137.8, 133.2, 132.0, 129.1, 128.1 (x2), 123.7, 122.5, 114.1 (x2), 69.1, 55.3, 27.7 (x 3), 24.3. cis-isomer (minor, selected peak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com