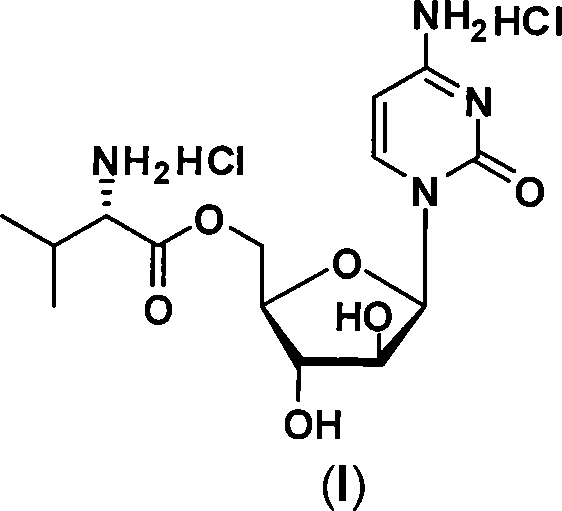
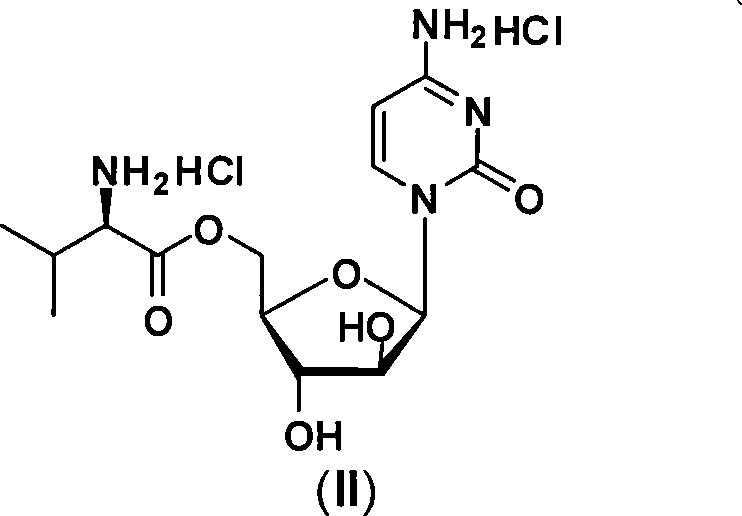
Alexan 5'-O-amino acid ester hydrochloride and preparation method thereof
A technology of valyl cytarabine hydrochloride and phenylalanyl cytarabine hydrochloride, applied in the field of medicine, can solve the problem of poor permeability of small intestine membrane, poor permeability of cytarabine membrane, poor permeability of cytarabine Cytarabine oral bioavailability and other issues to achieve the effect of improving membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
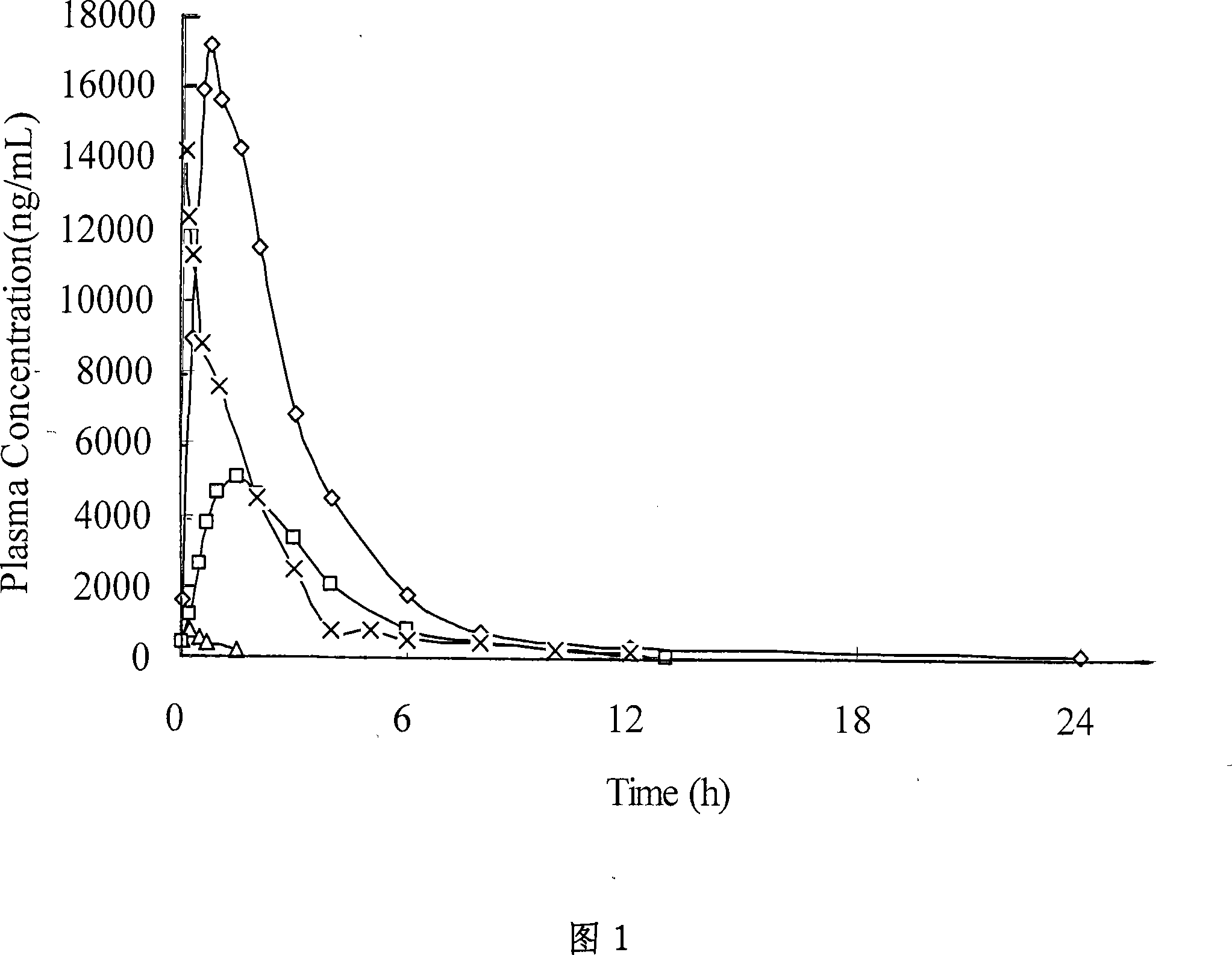
Image
Examples
Embodiment 1
[0030] N-tert-butoxyformyl-L-valine reacts with carbonyldiimidazole, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane or cyclohexane, the reaction temperature is from 0°C to the boiling point of the solvent, preferably 20-60°C, the reaction The time is 110 minutes; the reaction solution is slowly added dropwise to the mixed solution of compound VIII, 4-dimethylaminopyridine, triethylamine and N,N-dimethylformamide. After the addition is complete, the reaction is continued for 1-2 hours at a temperature of 50-100°C, preferably 60-90°C. The low boiling point solvent was evaporated under reduced pressure, the remaining N,N-dimethylformamide solution was adjusted to pH 7.69 with glacial acetic acid, N,N-dimethylformamide was evaporated under reduced pressure, and the residue was dissolved in ethyl acetate Dilute and wash with distilled water and saturated brine successively. The organic layer was concentrated to a certain extent, palladium carbon was added and c...
Embodiment 2
[0032] N-tert-butoxyformyl-D-valine reacts with carbonyldiimidazole, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane or cyclohexane, the reaction temperature is from 0°C to the boiling point of the solvent, preferably 20-60°C, the reaction The time is 110 minutes; the reaction solution is slowly added dropwise to the mixed solution of compound VIII, 4-dimethylaminopyridine, triethylamine and N,N-dimethylformamide. After the addition is complete, the reaction is continued for 1-2 hours at a temperature of 50-100°C, preferably 60-90°C. The low boiling point solvent was evaporated under reduced pressure, the remaining N,N-dimethylformamide solution was adjusted to pH 7.69 with glacial acetic acid, N,N-dimethylformamide was evaporated under reduced pressure, and the residue was dissolved in ethyl acetate Dilute and wash with distilled water and saturated brine successively. The organic layer was concentrated to a certain extent, palladium carbon was added and c...
Embodiment 3
[0034] N-tert-butoxyformyl-L-isoleucine reacts with carbonyldiimidazole, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane or cyclohexane, and the reaction temperature is from 0°C to the boiling point of the solvent, preferably 20-60°C, The reaction time was 110 minutes; the reaction solution was slowly added dropwise to the mixed solution of compound VIII, 4-dimethylaminopyridine, triethylamine and N,N-dimethylformamide. After the addition is complete, the reaction is continued for 1-2 hours at a temperature of 50-100°C, preferably 60-90°C. The low boiling point solvent was evaporated under reduced pressure, the remaining N,N-dimethylformamide solution was adjusted to pH 7.69 with glacial acetic acid, N,N-dimethylformamide was evaporated under reduced pressure, and the residue was dissolved in ethyl acetate Dilute and wash with distilled water and saturated brine successively. The organic layer was concentrated to a certain extent, palladium carbon was added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com