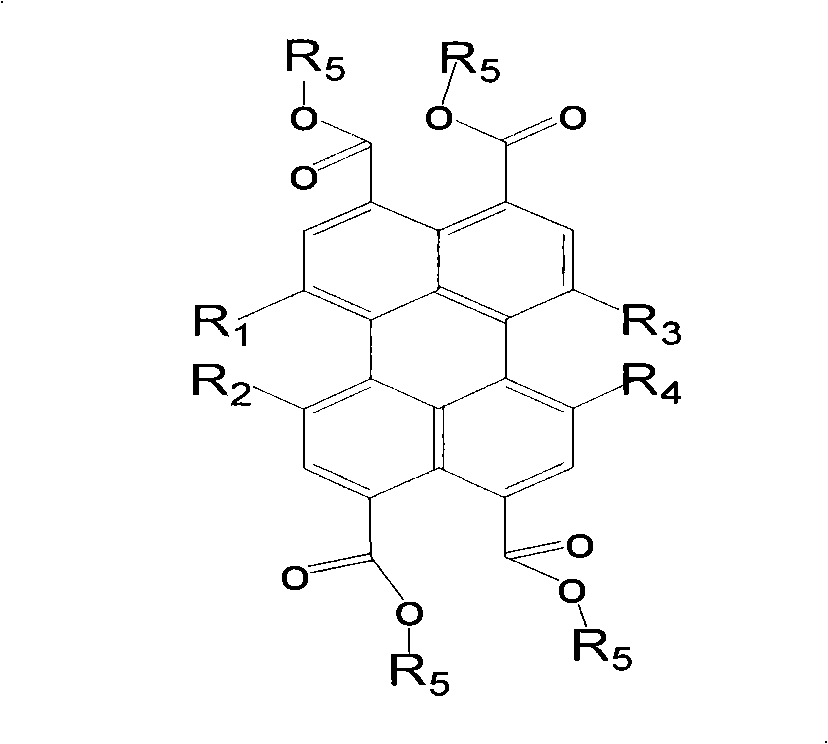
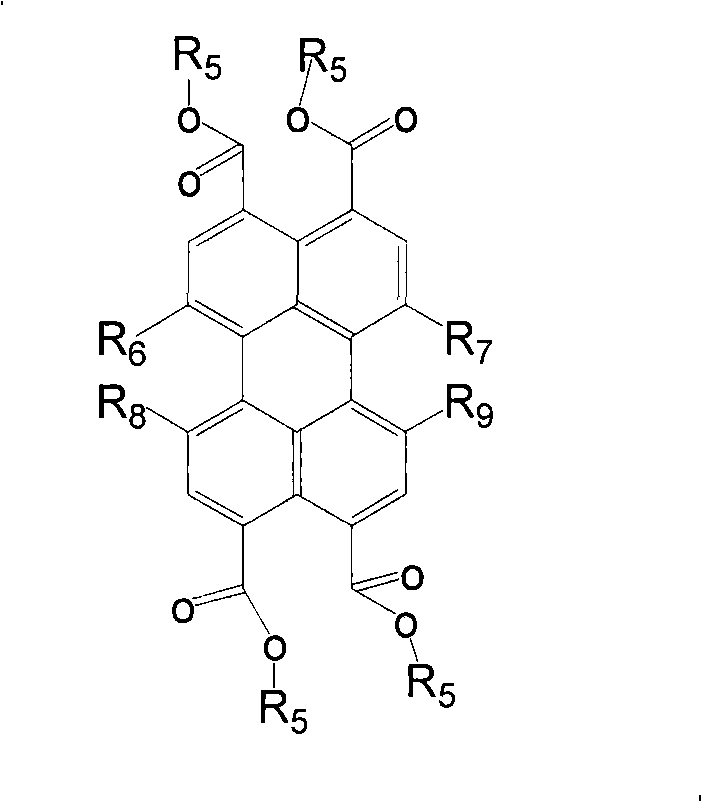
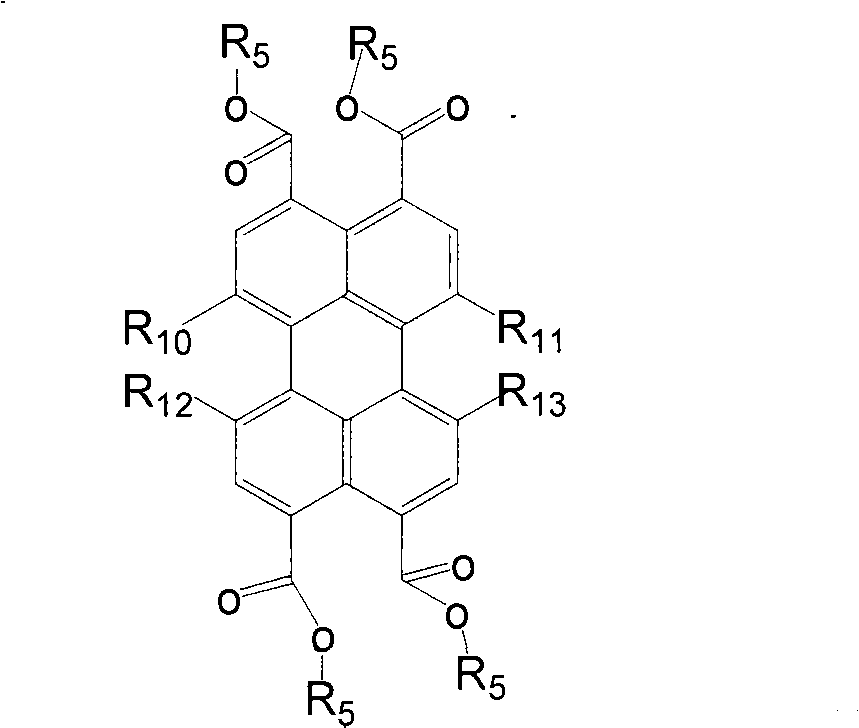
Trifluoromethyl substituted perylene bis diimines and preparation method thereof
A technology of perylene bis-diimide and trifluoromethyl, which is applied in the field of organic chemical industry and fine chemical industry to achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 1,7-ditrifluoromethyl-N,N'-dibutylperylene bisdiimide
[0035]
[0036] The synthetic route of this compound is shown in the figure above, which is synthesized through the following four steps:
[0037] (1) Synthesis of a mixture of 1,7-dibromoperylenetetracarboxylate tetrabutyl ester and 1,6-dibromoperylenetetracarboxylate tetrabutyl ester, add 820mg perylenetetracarboxylate (1.24mmol), 306mg to a 100mL two-neck bottle K 2 CO 3 (2.2mmol), 60mL CH 2 Cl 2 , Add 4mL liquid bromine (78.4mmol), keep the system at 10℃ under stirring, react for 2h, pass through a silica gel column to obtain 1,7-dibromoperylenetetracarboxylic acid tetrabutyl ester and 1,6-dibromoperylenetetracarboxylic acid tetrabutyl ester 600mg of ester mixture, 60% yield, API-ES-MS, [M+Na] + : 831(m / z); 1 H-NMR (400Mz, CDCl3): δ = 0.85-1.85 (m, 28H), 4.35 (m, 8H), 8.03 (m, 2H, perylene ring), 8.31 (m, 2H, perylene ring), 8.83 (m , 2H, perylene ring); 13 C-NMR(100MHz, CDCl 3 ): δ=14.0...
Embodiment 2
[0043] Example 2: Preparation of tetrabutyl 1-bromoperylenetetracarboxylate
[0044]
[0045] Add 3.0g tetrabutyl perylenetetracarboxylate (4.6mmol) to a 100mL two-necked bottle, 3.0g K 2 CO 3 (22mmol), 50mL CH2Cl2, add 3mL liquid bromine (59mmol), while stirring, keep the system at 20℃, react for 1.5h, and pass through a silica gel column to obtain 900mg of 1-bromoperylenetetracarboxylic acid tetrabutyl ester with a yield of 27%. API- ES-MS, [M+Na] + : 753(m / z); 1 H-NMR (400Mz, CDCl3): δ = 0.85-1.85 (m, 28H), 4.36 (m, 8H), 7.97 (d, 1H, perylene ring), 8.02 (d, 2H, perylene ring), 8.10 (m , 2H, perylene ring), 8.26 (s, 1H, perylene ring), 8.98 (d, 1H, perylene ring); 13 C-NMR(100MHz, CDCl 3 ): δ=13.8, 19.2, 19.3, 30.6, 65.4, 65.5, 65.7, 118.6, 121.6, 122.6, 127.3, 128.0, 129.2, 129.5, 130.0, 130.1, 130.2, 130.4, 130.8, 130.9, 131.6, 132.1, 132.4, 132.5, 137.4.
Embodiment 3
[0046] Example 3: Preparation of 1,6,7-tribromoperylenetetracarboxylic acid tetrabutyl ester and 1,6,7,12-tetrabromoperylenetetracarboxylic acid tetrabutyl ester
[0047]
[0048] Add 410mg tetrabutyl perylenetetracarboxylate (0.62mmol), 552mg K to a 25mL two-neck bottle 2 CO 3 (4mmol), 15mL of chlorobenzene, add 1mL of liquid bromine (19.6mmol), keep the system at 80°C under stirring, react for 48h, and pass through a silica gel column to obtain 110mg of 1,6,7-tribromoperylenetetracarboxylate tetrabutyl ester. Rate 19% API-ES-MS, [M+Na] + :908(m / z) and 1,6,7,12-tetrabromoperylenetetracarboxylic acid tetrabutyl ester 300mg, yield 50%, API-ES-MS, [M+Na] + : 986(m / z).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com