Aspergillus niger proline protein endopeptidase and preparation method thereof
A technology of Aspergillus niger proline and Aspergillus proline, which is applied in the field of genetic engineering and enzymology to achieve the effect of high efficiency and simple and feasible process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
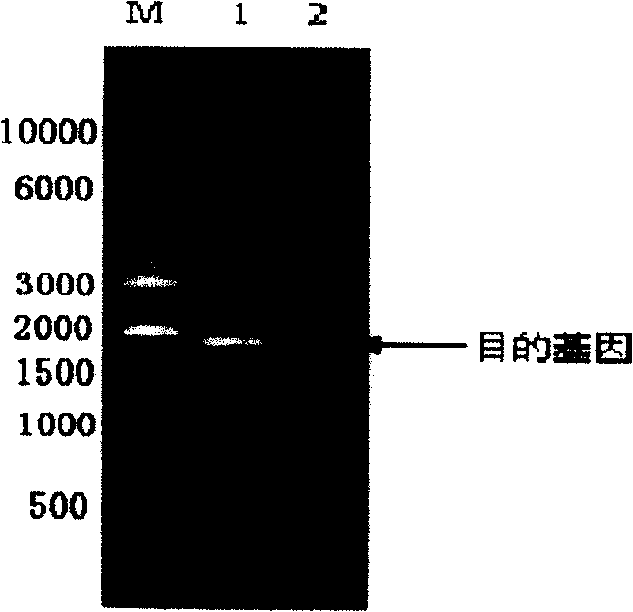
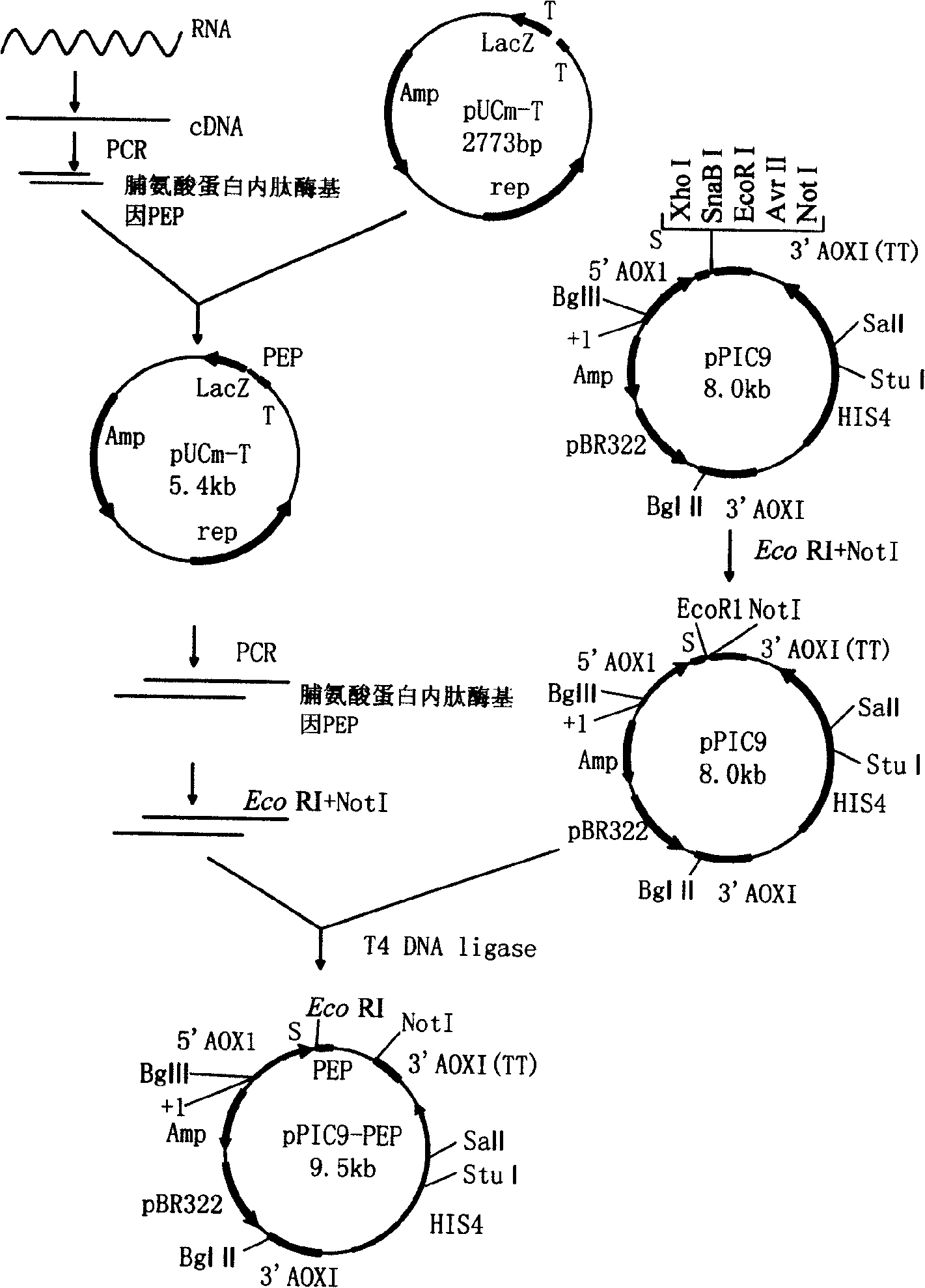
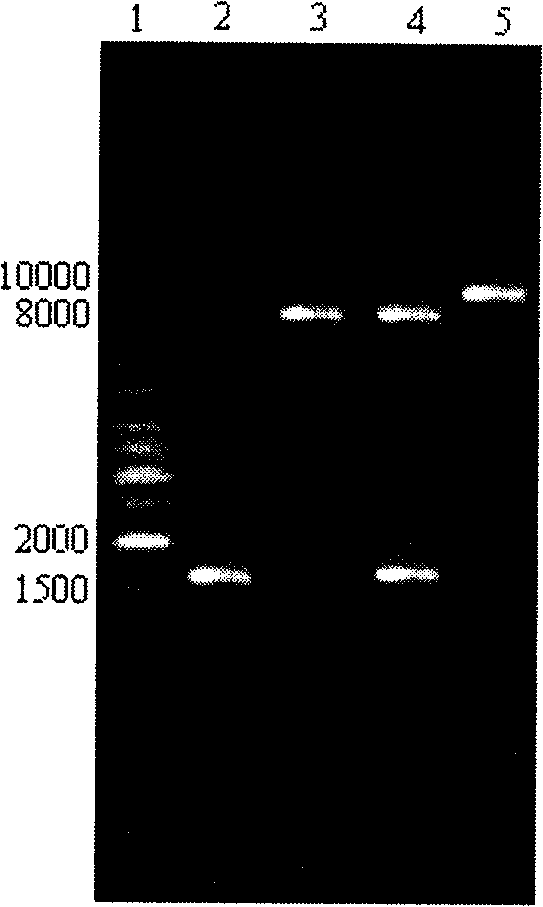
[0034] Below in conjunction with embodiment, the present invention is further described; Following embodiment is illustrative, not limiting, can not limit protection scope of the present invention with following embodiment.
[0035] First things first:
[0036] In the present invention, the term "Aspergillus niger proline endopeptidase" refers to the polypeptide having the sequence of SEQ ID No. 2 having the activity of Aspergillus niger proline endopeptidase. The term also includes variants of the sequence of SEQ ID No. 2 that have the same function as Aspergillus niger proline endopeptidase. These variations include (but are not limited to): deletions and insertions of several (usually 1-50, preferably 1-30, more preferably 1-20, and most preferably 1-10) amino acids and / or substitution, and addition of one or several (usually within 20, preferably within 10, more preferably within 5) amino acids at the C-terminal and / or N-terminal. For example, in the art, substitutions w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com