Method for identifying miRNA in blood serum of colon cancer patient by Solexa technology
A technology of microRNA and patient serum, applied in the field of biomedicine, can solve the problems of low throughput, lack of vigilance in diagnosing colon cancer, sensitivity and repeatability improvement, and achieve high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Solexa sequencing technology was used to sequence the serum samples of patients with colon cancer and analyze the expression levels of each contained microRNA. The specific steps are:
[0041] (1) Collect serum samples from patients with colon cancer.
[0042] (2) Trizol method (Invitrogen Company) extracted total RNA from 100ml of mixed serum of the above patients, concentrated RNA by isopropanol precipitation, and quantified it with a spectrophotometer (100ml serum can usually enrich about 10-20μg of total RNA).
[0043] (3) About 10-20 μg of serum total RNA from patients with colon cancer was used to detect and analyze RNA samples using Solexa sequencing technology. The specific plan is:
[0044] a. Apply PAGE electrophoresis to purify and obtain RNA molecules with a length less than 30nt. An adapter is added to both ends of the RNA molecule.
[0045] b. While using the Solexa chip to immobilize one end of the RNA adapter molecule, since the surface of ...
Embodiment 2
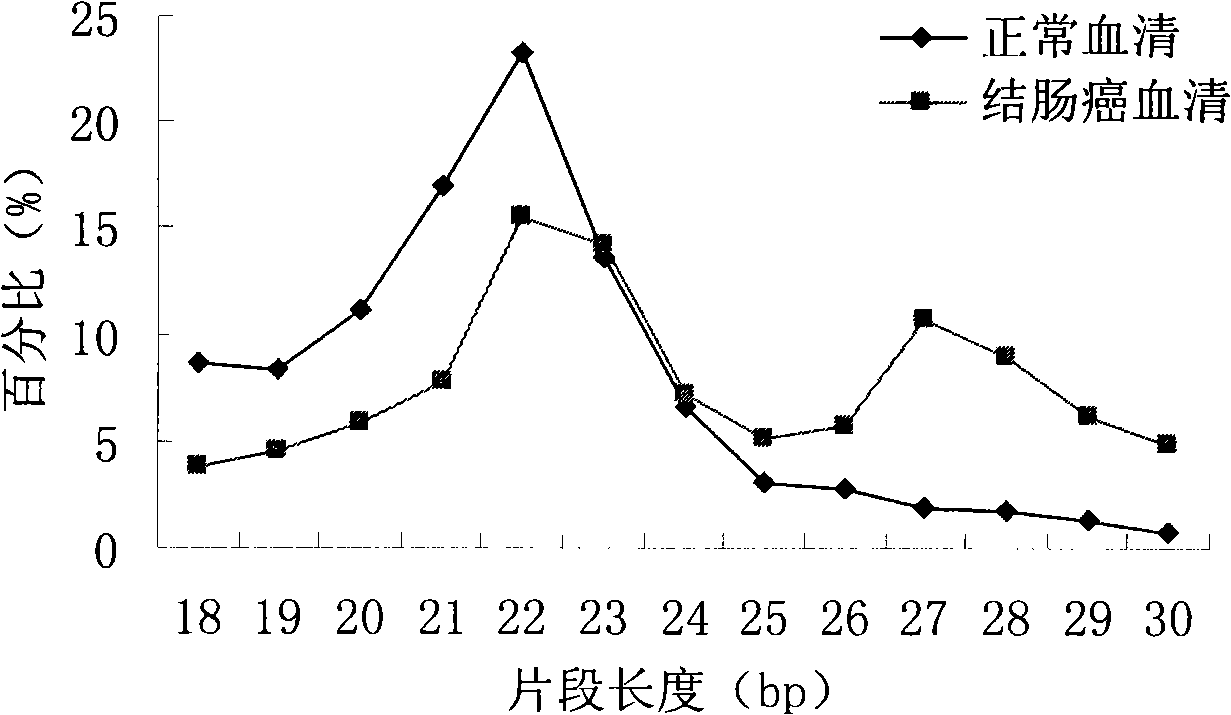
[0066] Example 2 Changes of microRNA in serum of patients with colon cancer compared with normal physiological state
[0067] Normal human serum microRNA was sequenced by Solexa technology, and the results are shown in Table 3.
[0068] Table 3 Solexa sequencing results of normal human serum
[0069]
[0070]
[0071]
[0072]
[0073]
[0074]
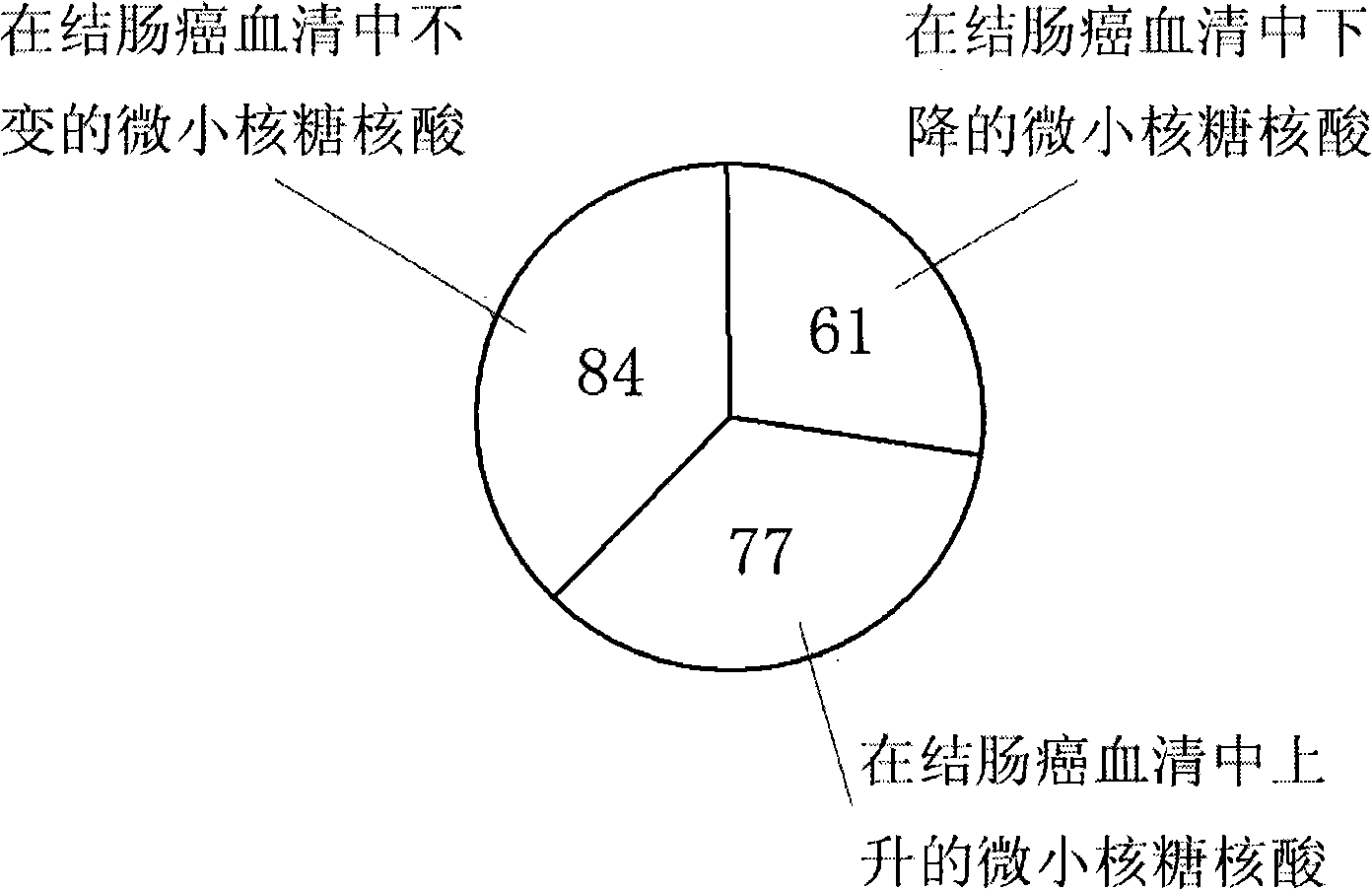
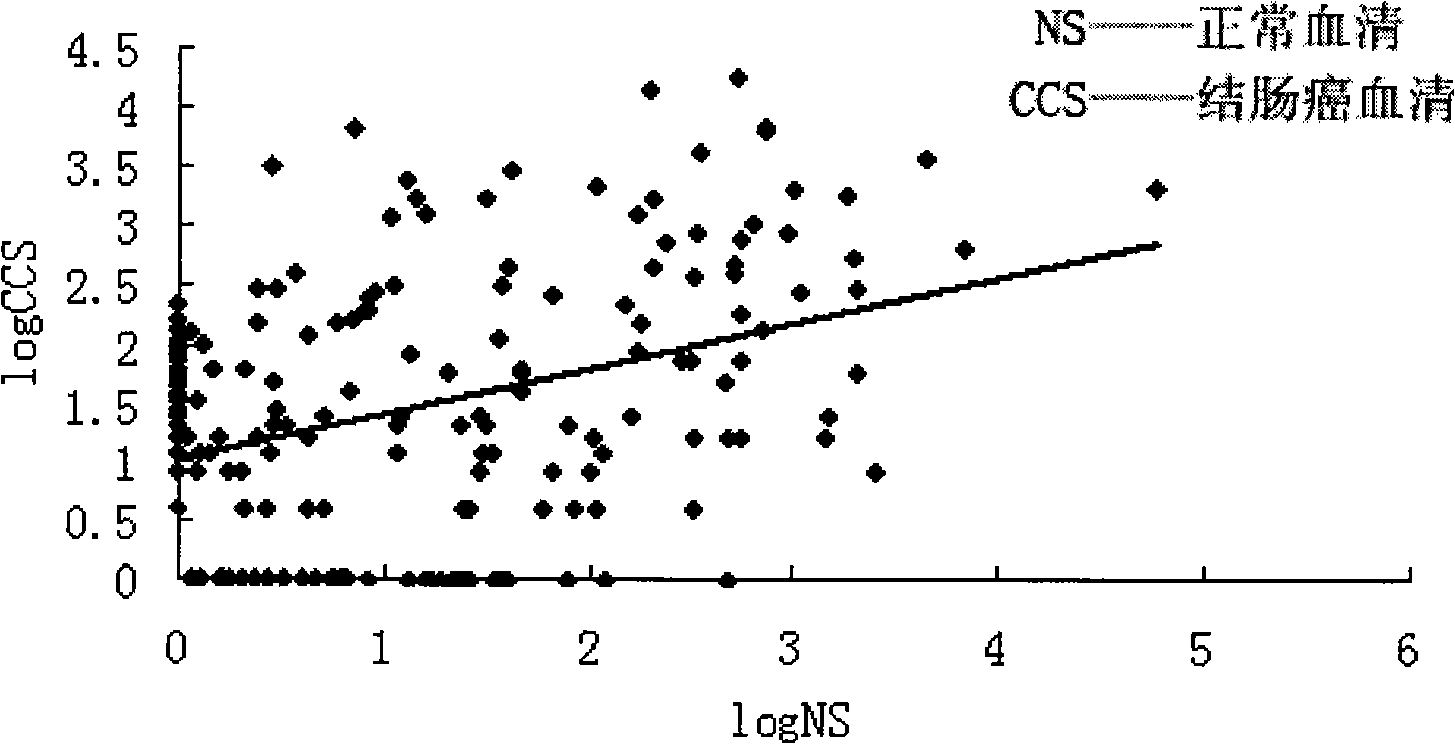
[0075] If the value of Solexa sequencing is less than 1 copy number, we consider it unreliable and default to zero after filtering. There are 222 microRNAs in the filtered colon cancer patient serum (see Table 2). There are 167 microRNAs (see Table 3) in the filtered normal human serum. We believe that when the change factor of the microRNA is greater than 5, and the difference in absolute value is greater than 20 copy numbers, then the microRNA is significantly changed, otherwise it is regarded as unchanged. According to this rule, we compared the microRNAs in the serum of patients with colon cancer and the serum...
Embodiment 3
[0076] Embodiment 3 is used for the making of the serum microRNA test kit of diagnosing colon cancer
[0077] The production process and operation process of the microRNA kit for diagnosing and predicting colon cancer are based on Solexa sequencing technology, combined with quantitative and semi-quantitative PCR technology.
[0078] Through the Solexa sequencing method, the serum microRNA expression profiles of normal people and colon cancer patients were first determined, and then a series of serum microRNAs with large expression levels and differences in colon cancer and normal physiological conditions were screened to predict whether colon cancer occurred. Cancer and diagnostic indicators of disease severity. Table 4 shows that compared with normal human serum, the significantly changed microRNAs in the serum of colon cancer patients can screen out a batch of serum microRNAs as molecular markers for the diagnosis of colon cancer.
[0079] Table 4 colon cancer patient's ser...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com