Preparation of condensed ring indole compounds
A technology for compounds and indoles, applied in the field of organic compound synthesis and preparation, can solve the problems of high toxicity of SnH, no industrial application value, low yield and the like, and achieve the effects of high industrial application value, convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
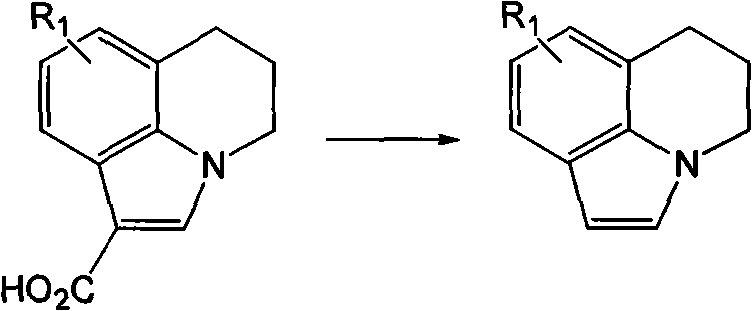
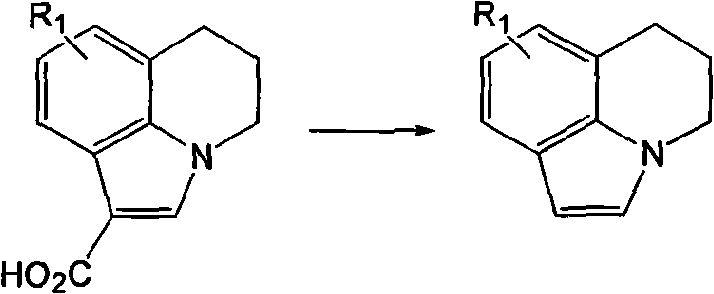
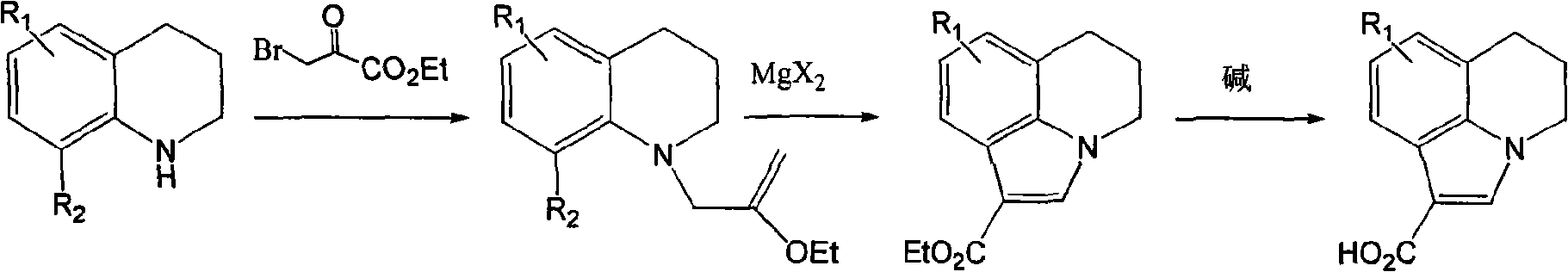
[0021] In the 250 ml three-necked bottle, a thermometer and a stirrer were installed, and the condensed ring indole carboxylic acid (R 1 =H, 0.1 mol), potassium chloride (0.1 mol), xylene (100 ml), then heated to 120° C. under reflux and stirred for 10 hours. Then the solvent was evaporated under reduced pressure, cooled to normal temperature, water (100 ml) was added, and the product was filtered and dried to obtain the product as a light yellow solid with a yield of 89% and a purity of 99.0 area% (HPLC).
Embodiment 2
[0023] In the 250 ml three-necked bottle, a thermometer and a stirrer are installed, and the condensed ring indole carboxylic acid (R 1 =5-CH 3 , 0.1mol), sodium chloride (0.12mol), toluene (100ml), and then heated to 100° C. under reflux and stirred for 15 hours. Then the solvent was evaporated under reduced pressure, cooled to normal temperature, water (110 ml) was added, and the product was filtered and dried to obtain the product as a light yellow solid with a yield of 85% and a purity of 99.5 area% (HPLC).
Embodiment 3
[0025] In the 250 ml three-necked bottle, a thermometer and a stirrer were installed, and the condensed ring indole carboxylic acid (R 1 =5-F, 0.1 mol), calcium chloride (0.2 mol), chlorobenzene (120 ml), then heated to 130° C. under reflux and stirred for 16 hours. Then the solvent was evaporated under reduced pressure, cooled to normal temperature, water (120 ml) was added, and the product was filtered and dried to obtain the product as a light yellow solid with a yield of 65% and a purity of 99.2 area% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com