IL-2 selective agonists and antagonists
A technology of IL-2 and IL-2R, applied in the fields of pharmacology and immunology, can solve problems such as not including, not describing the potential of low toxicity mutant proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1. Production of muteins in E. coli
[0102] Essentially as described in Kunkel TA, Roberts JD and Zakour RA, "Rapid and efficient site-specific mutagenesis without phenotypic selection", (1987), Methods Enzymol 154:367-382, using Primers for the desired mutated codons were subjected to site-directed mutagenesis to generate muteins. Briefly, human IL-2 cDNA containing restriction enzyme sites Bam HI and Xba I was subcloned into the M13 phage vector M13 mp19 (New England Biolabs, Beverly, MA) using the same sites. Wild-type IL-2 cDNA was obtained using polymerase chain reaction ("PCR") from a cDNA library generated from human cells induced with 12-myristate 13-acetate flubolate (10 ng / ml) for 24 hours. mRNA isolated from peripheral blood lymphocytes. The PCR primer used is the 5' end of the IL-2 open reading frame,
[0103] 5'-CCT CAA CTC CTG AAT TCA TGT ACA GGA TGC-3' (SEQ ID NO: 3);
[0104] and the 3' end of the IL-2 open reading frame,
[0105] 5'-GGA AG...
Embodiment 2
[0113] Example 2. Extraction and purification of IL-2 mutein from Escherichia coli
[0114] After 3 hours of induction, cells were collected by centrifugation at 10,000 xg. Recombinant IL-2 muteins were first refolded and purified by dispersing cells in 10 volumes (vol / wet weight) of sucrose / Tris / EDTA buffer (0.375M sucrose, 10 mM Tris / HCl pH 8.0, 1 mM EDTA). The dispersed cells were sonicated 3 times at 300W with 30 second rest intervals on an ice bath using a Missonix XL2020 model sonicator equipped with a 1 inch standard probe. The sonicated material was then centrifuged at 17,000 xg, 4°C for 20 minutes. The pellet, which should be white at this point, was washed by resuspending in sucrose / Tris / EDTA buffer and centrifuging once, resuspending in Tris / EDTA buffer (50 mM Tris / HCL pH 8.0, 1 mM EDTA) and centrifuging twice. times, finally resuspended in 10 volumes of 0.1M Tris / HCL, pH 8.0 buffer (at which time a sample was taken for gel analysis) and centrifuged at 17,000 xg f...
Embodiment 3
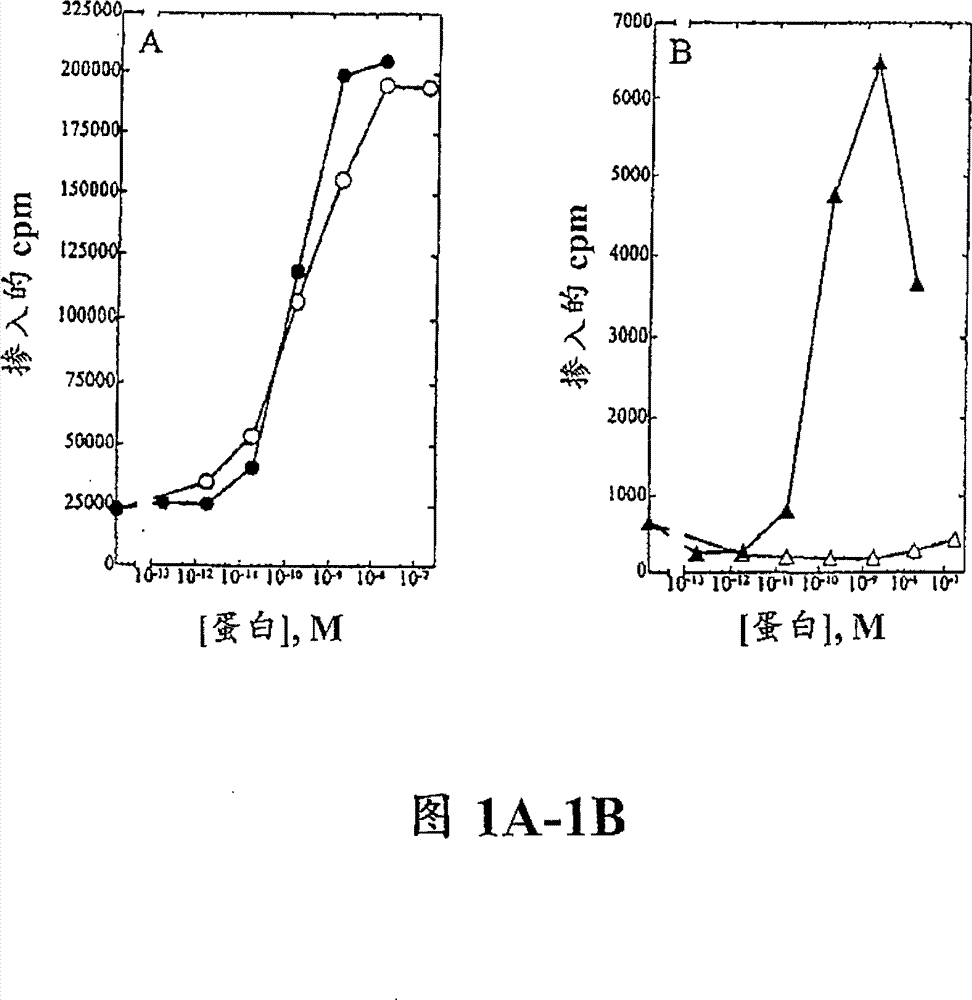
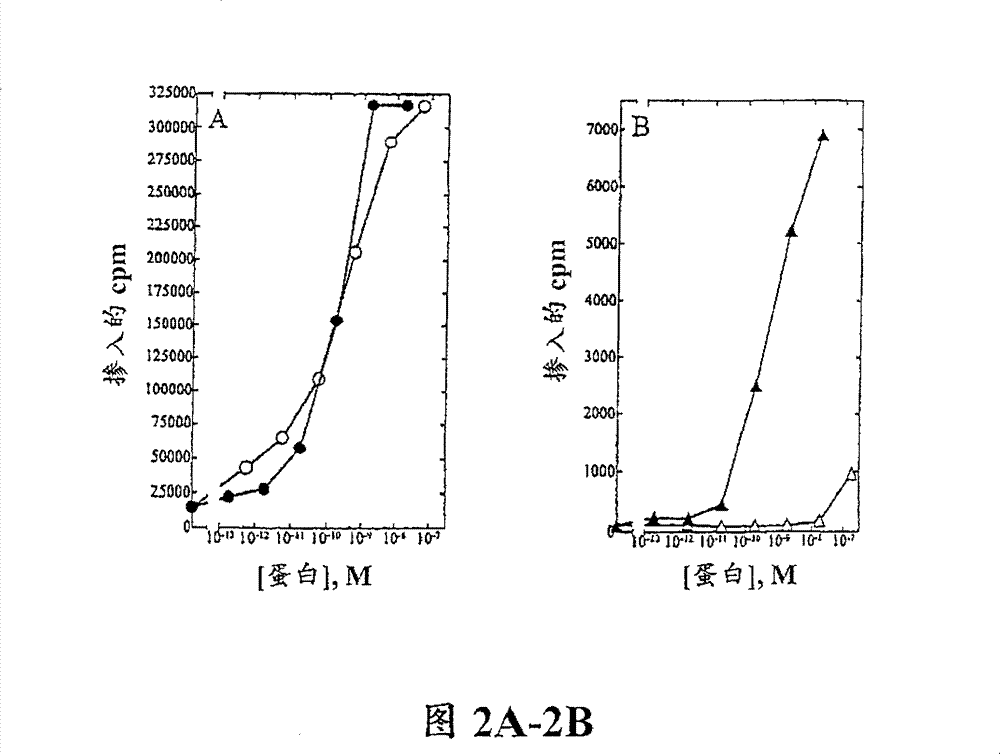
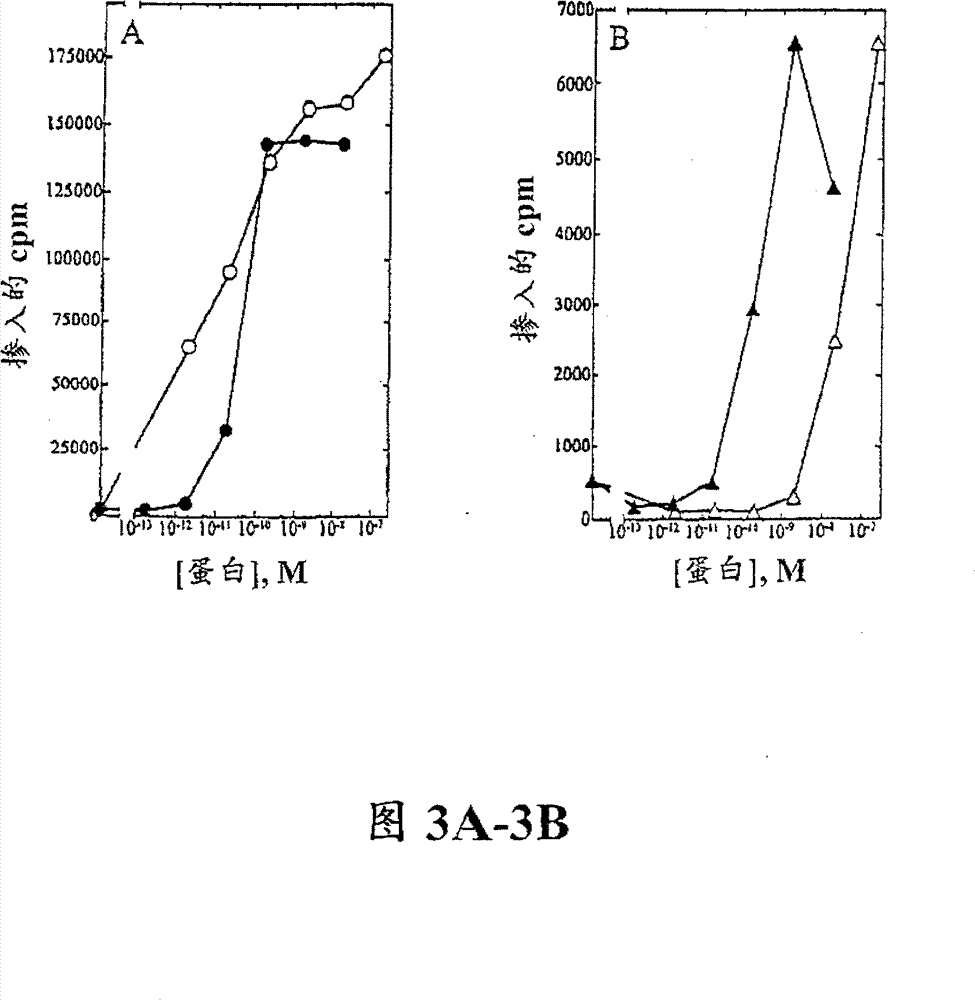
[0116] Example 3. T cell proliferation analysis
[0117] Peripheral blood mononuclear cells (PBMC) were isolated from approximately 100 mL of normal human blood (Irwin Memorial Blood Bank, San Francisco, CA) in cold Dulbecco's phosphate-buffered saline (Ca 2+ and Mg 2+ free; DPBS) at a 1:2 dilution. Ficoll-Paque (Pharmacia) was placed in the lower layer, and the samples were centrifuged to separate PBMCs, followed by extensive washing in cold DPBS. PHA blasts (activated T cells) were generated by the following method: 1×10 6 Cells / ml density Cells were resuspended in RPMI 1640 containing 10% fetal bovine serum (Hyclone) supplemented with 1% (w / v) of the following: L-glutamine, non-essential amino acids, sodium pyruvate and Antibiotic-antimycotic (RPMI medium). Phytohemagglutinin (PHA-P; Sigma) was added at a final concentration of 10 μg / mL, and the cells were incubated at 37°C, 5% CO 2 Cultured for 3 days. Cells were harvested and washed twice in DPBS, resuspended in RPM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com