Bifunctionan air electrode
An air electrode, dual-function technology, applied in the manufacture of such electrodes, dual-function air electrodes, secondary metal-air batteries, secondary metal hydride-air batteries, can solve problems such as long charging time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
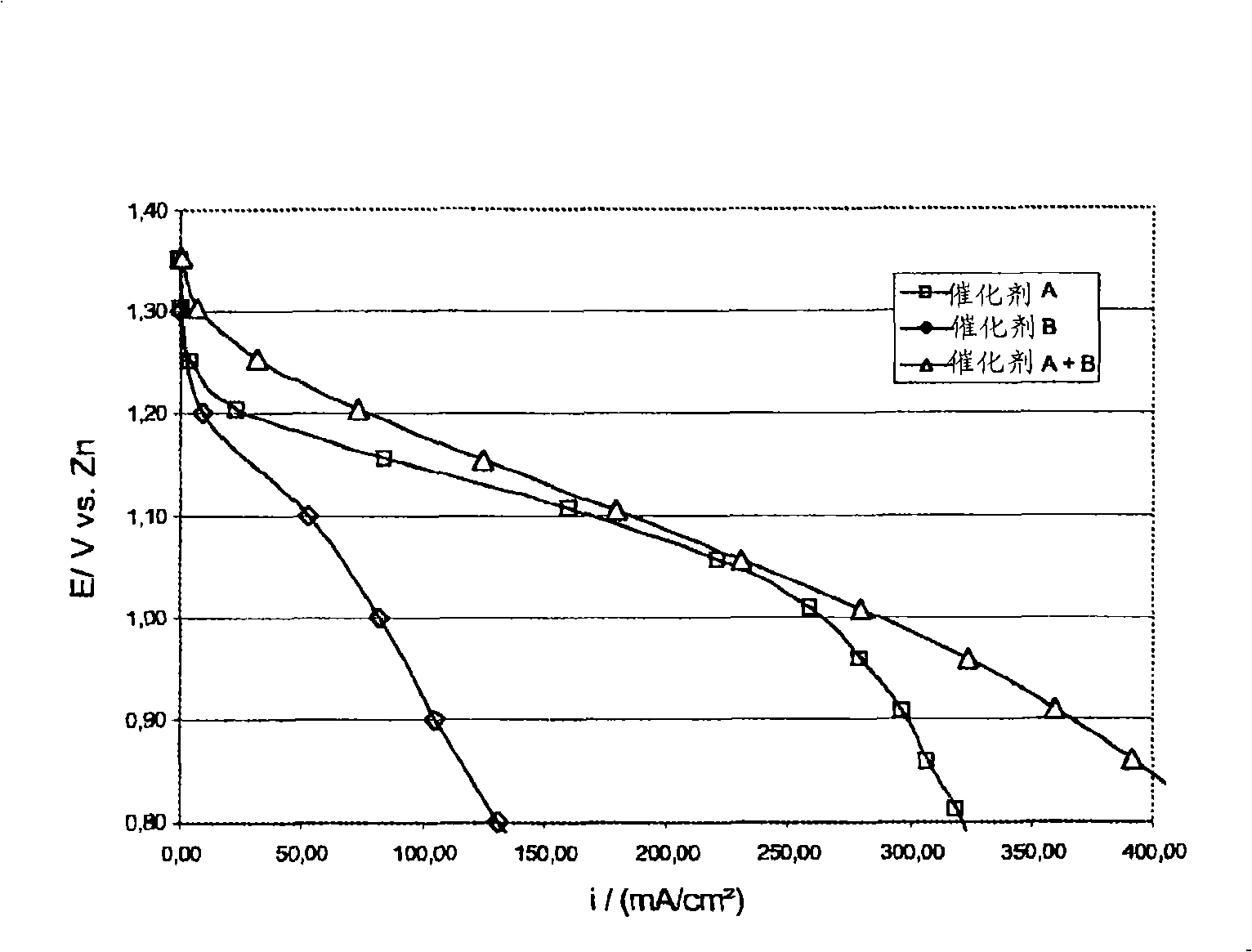
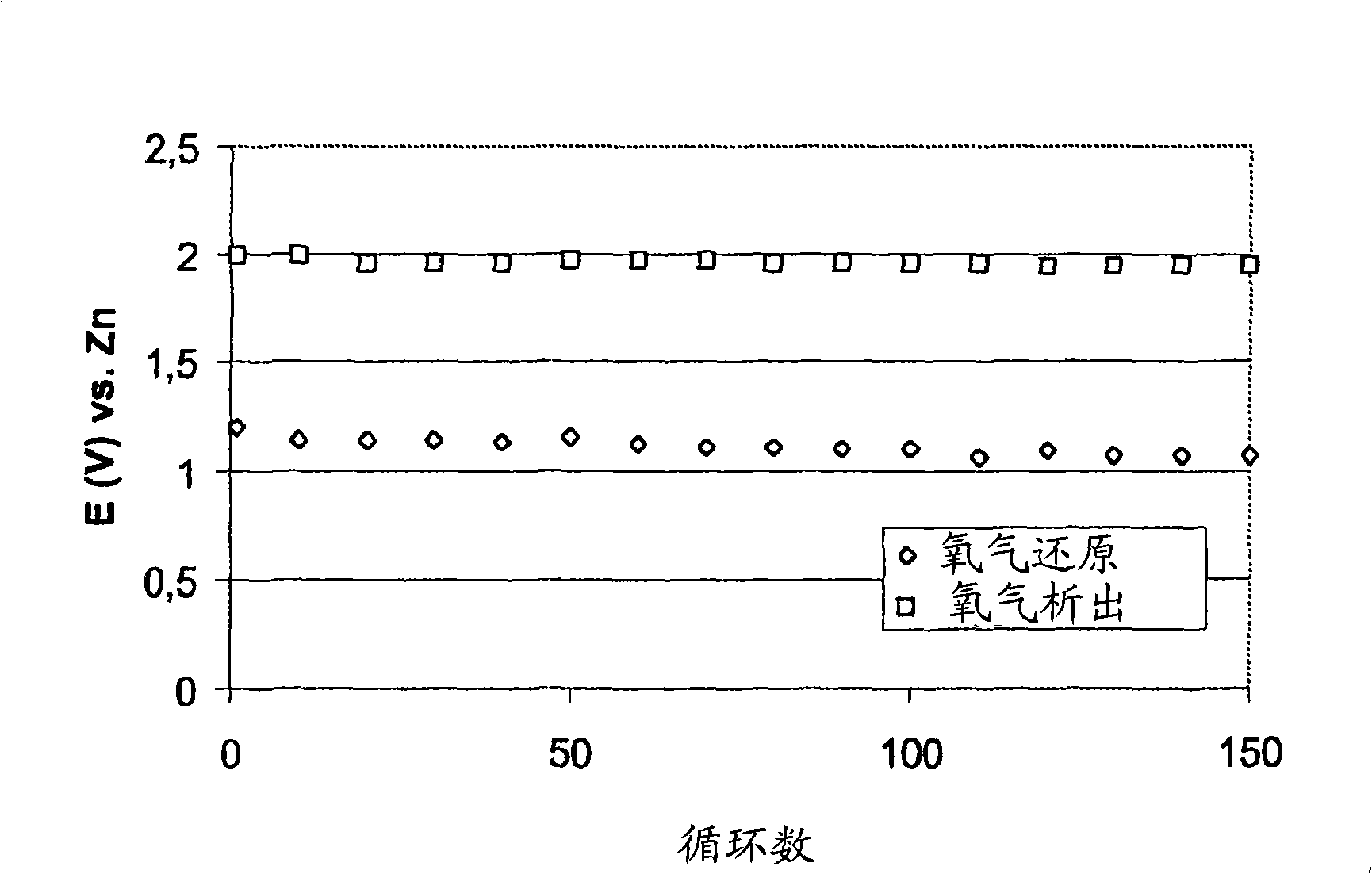
[0066] This example shows that the combined use of an oxygen reduction catalyst and a bifunctional catalyst increases the rate of oxygen reduction and the cycle life of the bifunctional electrode. Select MnSO 4 As an oxygen reduction catalyst, La 2 o 3 as a bifunctional catalyst.
[0067] Air electrodes were prepared using high surface area carbon, catalysts in powder form, and PTFE suspensions.
[0068] The active layer was prepared using 15 wt% PTFE (as a suspension (Aldrich) containing 60 wt% PTFE in water dispersion), 63.5 wt% high surface area carbon (XC500, Cabot Corporation) and an electrocatalyst: 13 wt% manganese sulfate (MnSO 4 , Prolabo) and 8.5wt% lanthanum oxide (La 2 o 3 , Merck). As a first step, the high-surface-area carbon was mixed with the two catalysts in water. Separately, the PTFE suspension and water were mixed. Then, the PTFE solution is added to the carbon solution and the material is mixed and coalesced into a slurry. The slurry was then mixe...
Embodiment 2
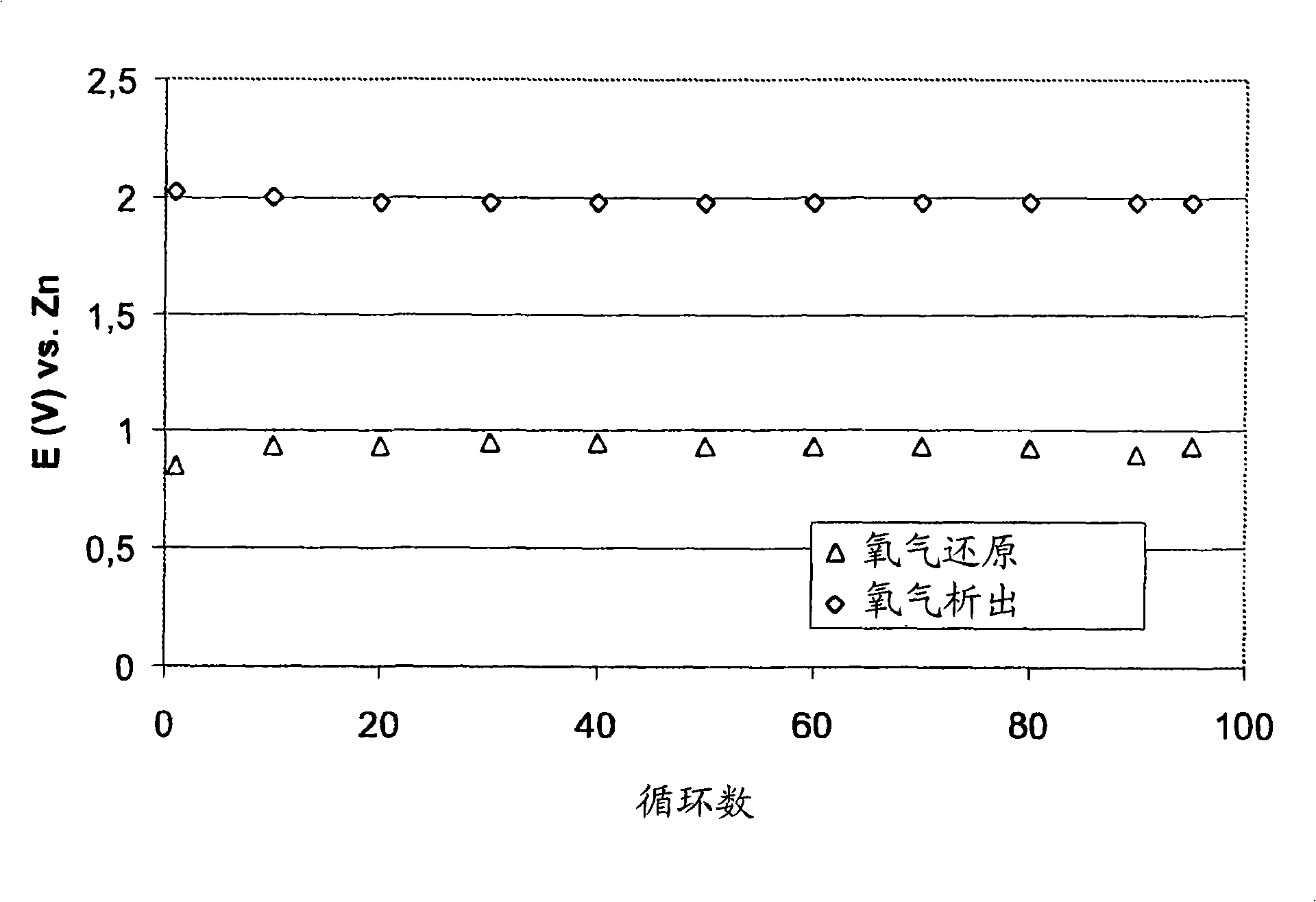
[0077] This example shows that when using MnO as an oxygen reduction catalyst 2 and La as a bifunctional catalyst 2 o 3 The activity and stability of the air electrode when combined. The air electrode was prepared using high surface area carbon, powdered catalyst and PTFE suspension.
[0078] The active layer was prepared using 15 wt% PTFE (as a suspension (Aldrich) containing 60 wt% PTFE in aqueous dispersion), 69 wt% high surface area carbon (XC500, Cabot Corporation) and an electrocatalyst: 8 wt% manganese oxide (MnO 2 , Merck) and 8wt% lanthanum oxide (La 2 o 3 , Merck). As a first step, the high-surface-area carbon was mixed with the two catalysts in water. Separately, the PTFE suspension and water were mixed. Then, the PTFE solution is added to the carbon solution and the material is mixed and coalesced into a slurry. The slurry was then mixed in an ultrasonic bath for 30 minutes. The slurry was then dried at 300°C for 3 hours to remove any surfactant. The drie...
Embodiment 3
[0085] This example shows how the amount of catalyst affects the activity of an air electrode.
[0086] Several electrodes were fabricated according to the electrode fabrication procedures described in Examples 1 and 2, with varying amounts of oxygen reduction catalyst and bifunctional catalyst.
[0087] For all electrodes, high surface area carbon (XC500) and 20 wt% PTFE in AL were used. GDL was made according to the description given in Examples 1 and 2.
[0088] Table 1 shows the effect of catalyst amount on electrode stability.
[0089] Table 1: Discharge voltage and charge / discharge stability of bifunctional air electrodes
[0090] wt% /
MnSO 4
wt% /
MnO 2
wt% /
La 2 o 3
Capacity / Ah (1)
vs. Zn (2)
1.6
0
8
75
0.98
13
0
8.5
375
1.18
40
0
8
3.1
1.18
12
0
1.6
31.3
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com