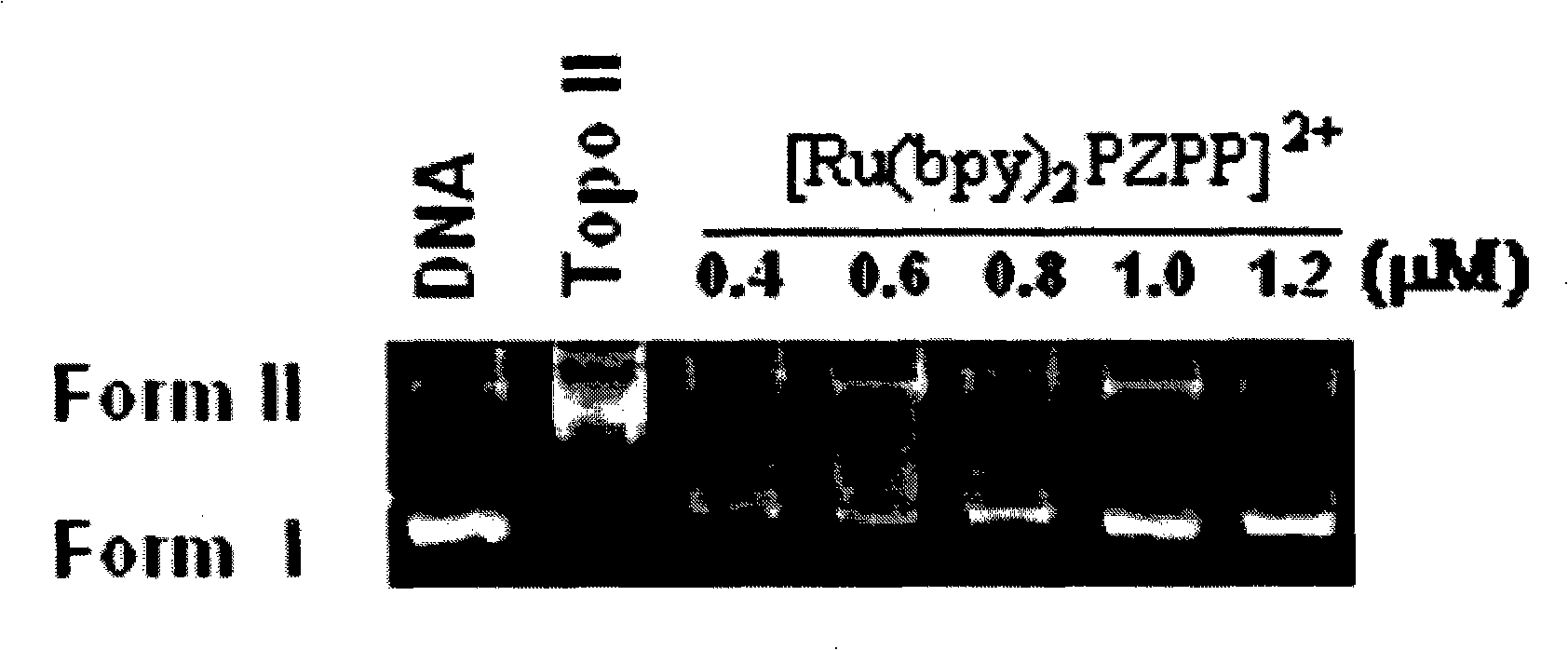Ruthenium complexes for restraining DNA from topologizing isomerase , preparation method and application thereof
A technology of topoisomerase and ruthenium complexes, which is applied in the field of ruthenium complexes inhibiting DNA topoisomerase and its preparation, can solve the problems of low specificity, poor solubility, complex structure, etc., and achieve simple preparation methods, The effect of improving water solubility and warming reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
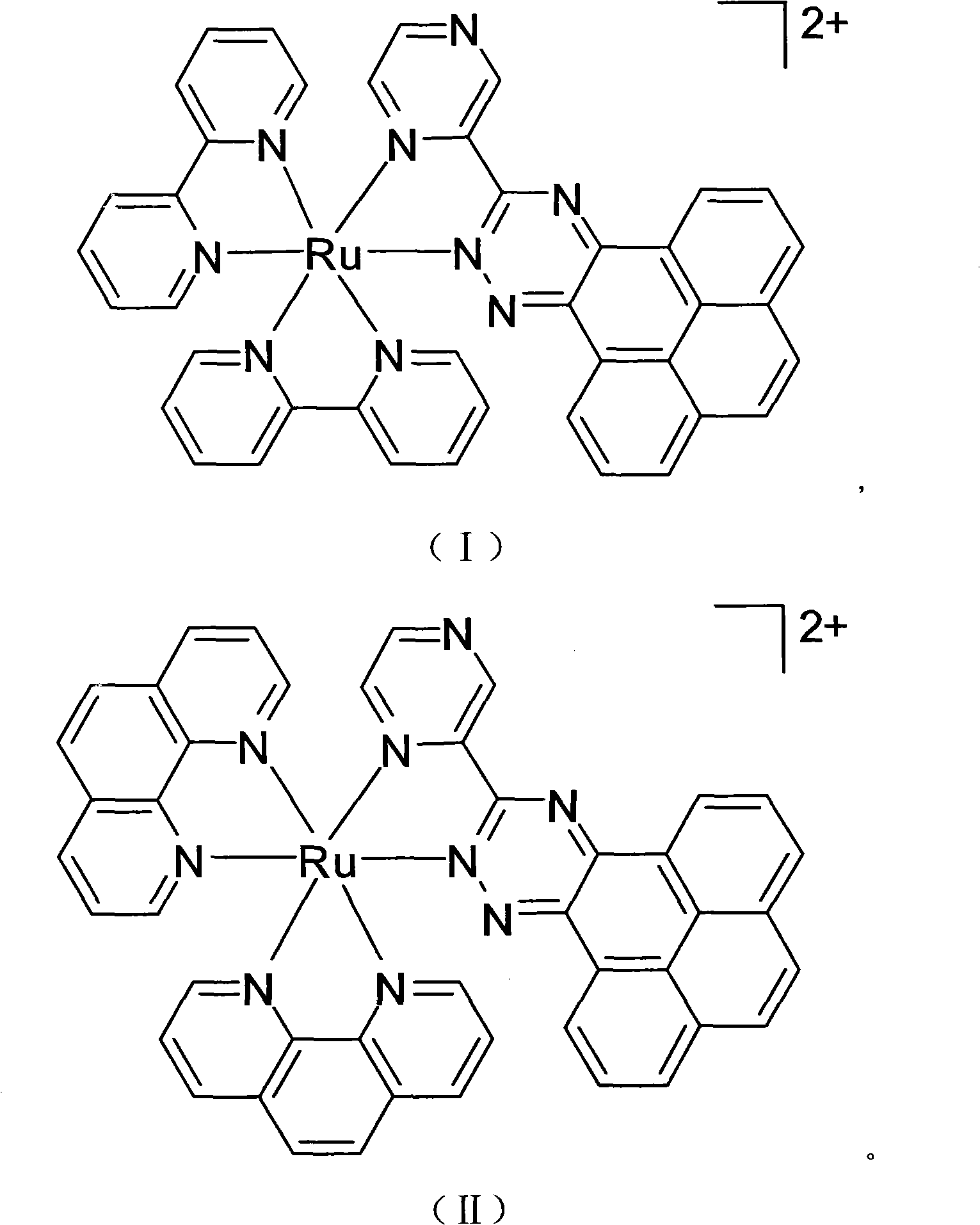
[0031] (1) Preparation of ligand 3-(2-pyrazinyl)-1,2,4-triazino[5,6-e]pyrene (PZPP):
[0032] 2-Pyrazine imidine (1.1 g, 8 mmol) and pyrenedione (1.8 g, 8 mmol) were heated under reflux in ethanol for 4 hours. Cooling to room temperature gave a yellow precipitate. The precipitate was collected and dried in vacuo as a yellow solid, yield 76%.
[0033] Elemental Analysis C 21 h 11 N 5 (Molecular weight 333), experimental value: C 75.69, H 3.28, N 21.03%; theoretical value: C 75.68, H 3.30, N 21.02%. FAB-MS: m / z=334 (C 21 h 11 N 5 333).
[0034] (2) Preparation of [Ru(bpy) 2 (PZPP)]Cl 2 : After bis(2,2'-bipyridine)-dichloro-ruthenium(II) dihydrate (1.0g, 2mmol) and PZPP (0.6g, 2mmol) were refluxed in ethylene glycol for 10 hours, NaClO was added 4 Aqueous solution, precipitated red solid. The crude product dried by suction filtration is separated and purified by alumina column chromatography, then dissolved in acetone, added tetrabutylammonium chloride, a red solid is...
Embodiment 2
[0037] (1) Preparation of ligand 3-(2-pyrazinyl)-1,2,4-triazino[5,6-e]pyrene (PZPP):
[0038] 2-Pyrazine imidine (1.1 g, 8 mmol) and pyrenedione (1.8 g, 8 mmol) were heated under co-flow in ethanol for 4 hours. Cooling to room temperature gave a yellow precipitate. The precipitate was collected and dried in vacuo as a yellow solid, yield 76%.
[0039] Elemental Analysis C 21 h 11 N 5 (Molecular weight 333), experimental value: C 75.69, H 3.28, N 21.03%; theoretical value: C 75.68, H 3.30, N 21.02%. FAB-MS: m / z=334 (C 21 h 11 N 5 333).
[0040] (2) Preparation of [Ru(phen) 2 (PZPP)]Cl 2 : After bis(1,10-phenanthroline)-dichloro-ruthenium dihydrate (II) (1.1g, 2mmol) and PZPP (0.6g, 2mmol) were refluxed in ethylene glycol for 10 hours, NaClO was added 4 Aqueous solution, precipitated red solid. The crude product dried by suction filtration was separated and purified by alumina column chromatography, then dissolved in acetone, added tetrabutylammonium chloride, and a ...
Embodiment 3
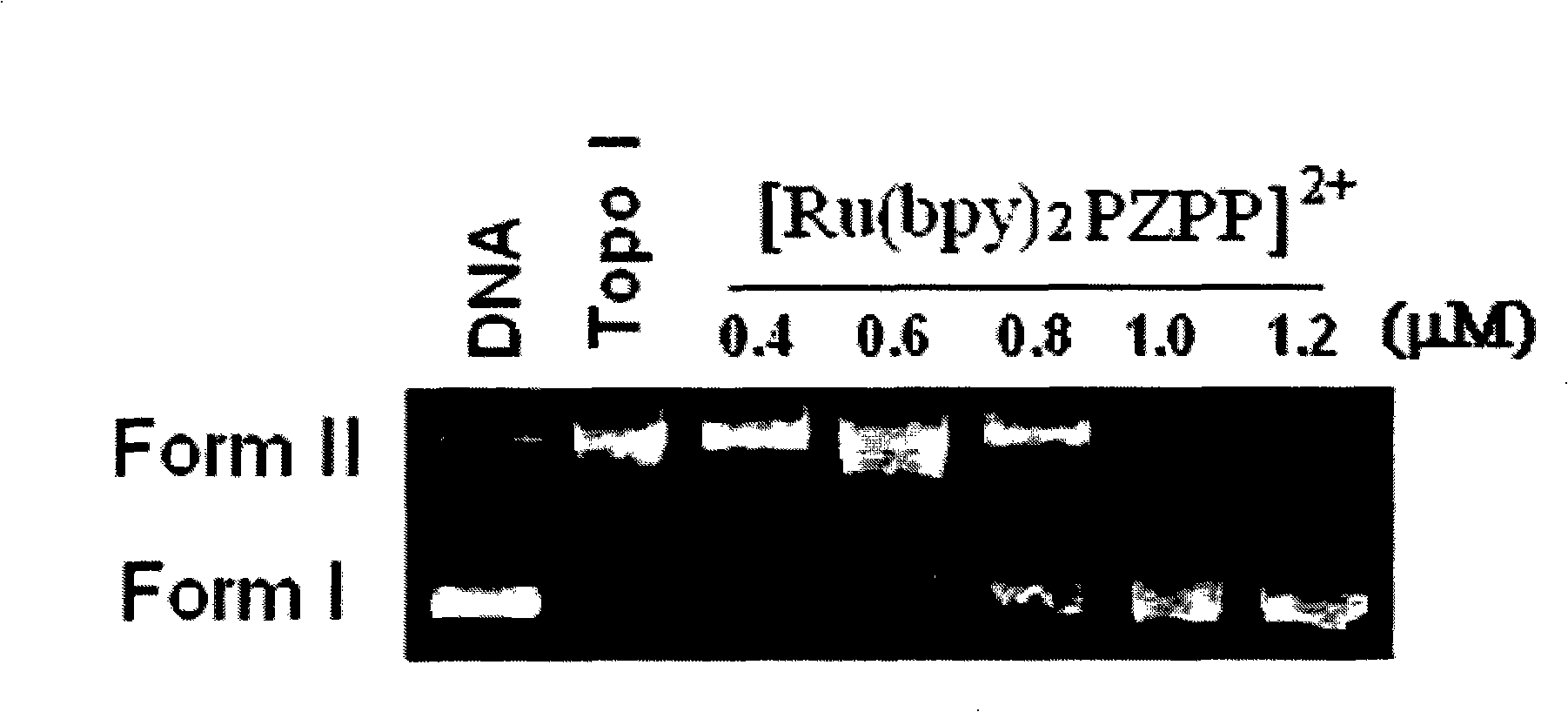
[0042] Example 3 Topoisomerase Inhibition Experiment of Ru(II) Complexes
[0043] According to the method of drug inhibition topoisomerase unwinding test, the inhibitory ability was judged, and the compound was reacted with pBR322 DNA and topoisomerase in an appropriate buffer, and the reaction mixture was incubated at 37°C for a certain period of time. The reaction was terminated by adding reaction termination solution. On 0.9% agar enamel (TBE) gel, electrophoresis under constant voltage condition of 75V. The gel was stained with 1 μg / mL EB solution and photographed under UV light. The complex concentration that inhibits 50% of Topo I or Topo II activity was defined as IC 50 . The experimental results show that the complex is a dual inhibitor of Topo I and Topo II, showing a very good inhibitory ability, IC 50 values less than 1 µM, see figure 1 and figure 2 , in the figure, the plasmid DNA is expressed as a closed-loop supercoiled Form I band; a certain amount of T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com