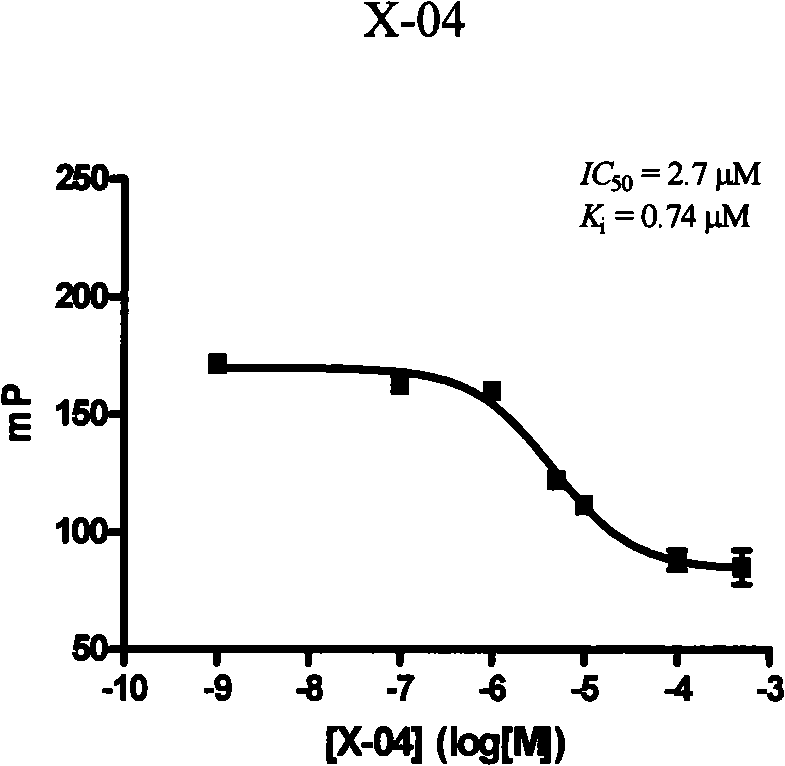Thiazole couplet pyrazolone series compound and application of the same as Bcl-2 family protein antagonist
A technology of thiazole bipyrazolone and compound is applied to thiazole bipyrazolone compound and its application field as Bcl-2 family protein antagonist, and can solve the problem of decreasing fluorescence polarization value and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: Preparation of (E)-4-(2-(4-chlorophenyl)hydrazono)-3-methyl-1-(4-phenylthiazol-2-yl)-1H-pyrazole- 5(4H)-keto
[0092] 1. Synthesis of (Z)-2-(2-(4-chlorophenyl)hydrazono)-3-oxobutanoic acid ethyl ester (formula II-05)
[0093]
[0094]
[0095] Suspend 1.276g (10mmol) of 4-chloroaniline (Formula I-05) in 8mL of water, slowly inject 5mL of concentrated HCl at about room temperature, and slowly inject 0.690g (10mmol) of A 5mL aqueous solution of sodium nitrate was directly filtered into a 25mL ethanol solution containing 1.28mL (10mmol) ethyl acetoacetate and 8g (97.5mmol) sodium acetate, a yellow solid was precipitated, suction filtered, washed with water, and recrystallized from absolute ethanol to obtain 1.914 g of yellow needle-like crystals (71% yield).
[0096] After testing, the structure is correct, and the test results are as follows: mp 80-84°C; IR (KBr): v 2994, 1704, 1617, 1587, 1528, 1495, 1474, 1456, 1419, 1396, 1372, 1330, 1276, 1205, 116...
Embodiment 2
[0107] Embodiment 2: formula X-04, X-05, X-06, X-07, X-09, X-10, X-13, X-19, X-20, X-24, X-26, X Synthesis of -27, X-31, X-32, X-33, X-34, X-35, X-37, X-39, X-40, X-41 compounds
[0108] One, the synthesis of formula II-01-04 and formula II-06-17
[0109]
[0110]
[0111]
[0112] Under conditions similar to those of the compound of formula II-05 in Example 1, compounds of formula II-01-04 and formula II-06-17 were obtained from the corresponding formula I-01-04 and formula I-06-17: ( Z)-2-(2-phenylhydrazono)-3-oxobutanoic acid ethyl ester (formula II-01); (Z)-2-(2-(4-fluorophenyl)hydrazono) -3-oxobutanoic acid ethyl ester (formula II-02); ); (Z)-2-(2-(3-chlorophenyl)hydrazono)-3-oxobutanoic acid ethyl ester (formula II-04); (Z)-2-(2-(3, 4-dichlorophenyl) hydrazono)-3-oxobutanoic acid ethyl ester (formula II-06); (Z)-2-(2-(2-methylphenyl) hydrazono)-3 -Ethyl oxobutyrate (formula II-07); (Z)-2-(2-(3-methylphenyl)hydrazono)-3-oxobutanoic acid ethyl ester (formula I...
Embodiment 3
[0122] Embodiment 3: Thiazole bipyrazolone compounds to Bcl-x L Fluorescence polarization detection of inhibition
[0123] Glutathione-S-transferase (GST) fused Bcl-x L (GST-Bcl-x L) protein was expressed in Escherichia coli BL21, and was separated and purified by glutathione sepharose column affinity chromatography; the fluorescently labeled polypeptide was Bid BH3 polypeptide labeled with 5-FAM at the N-terminal (sequence: 5-FAM -QEDIIRNIARHLAQVGDSMDR); compounds X-04, X-05, X-06, X-07, X-09, X-10, X-13, X-19, X-20, X-21, X-24, X -26, X-27, X-31, X-32, X-33, X-34, X-35, X-37, X-39, X-40, X-41 were prepared by the above method, and the rest of the compounds were purchased from From SPECS Corporation.
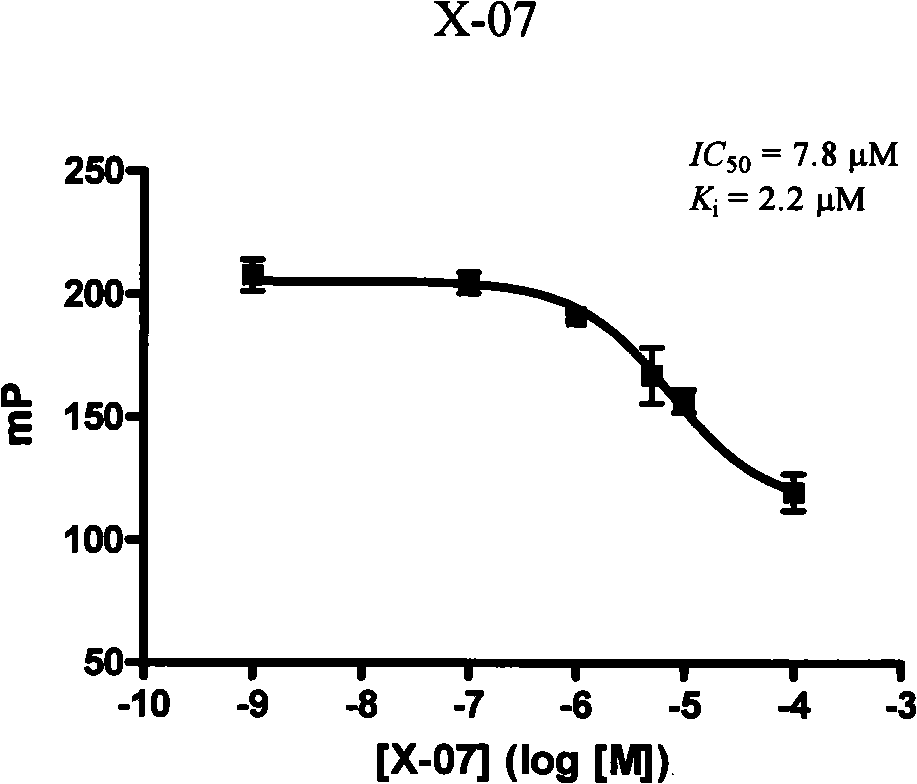
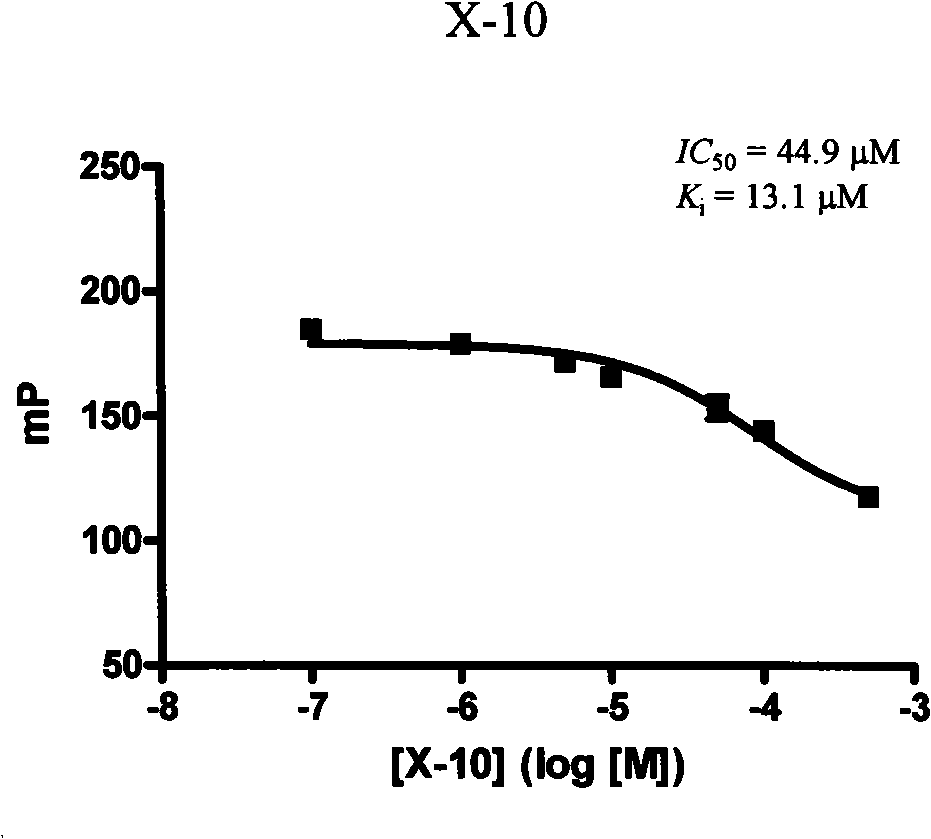
[0124] One, compound formula X-04, the inhibition constant determination of X-07 and X-10
[0125] In test buffer (1× phosphate buffered saline, containing 0.02% (w / v) NaN 3 ) by adding GST-Bcl-x L 1×PBS solution of different concentrations of the compound and DMSO solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com