Process for the preparation of S-(+)-clopidogrel by optical resolution
A clopidogrel, optical separation technology, applied in organic chemistry, blood diseases, extracellular fluid diseases, etc., can solve problems such as difficult crystallization and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
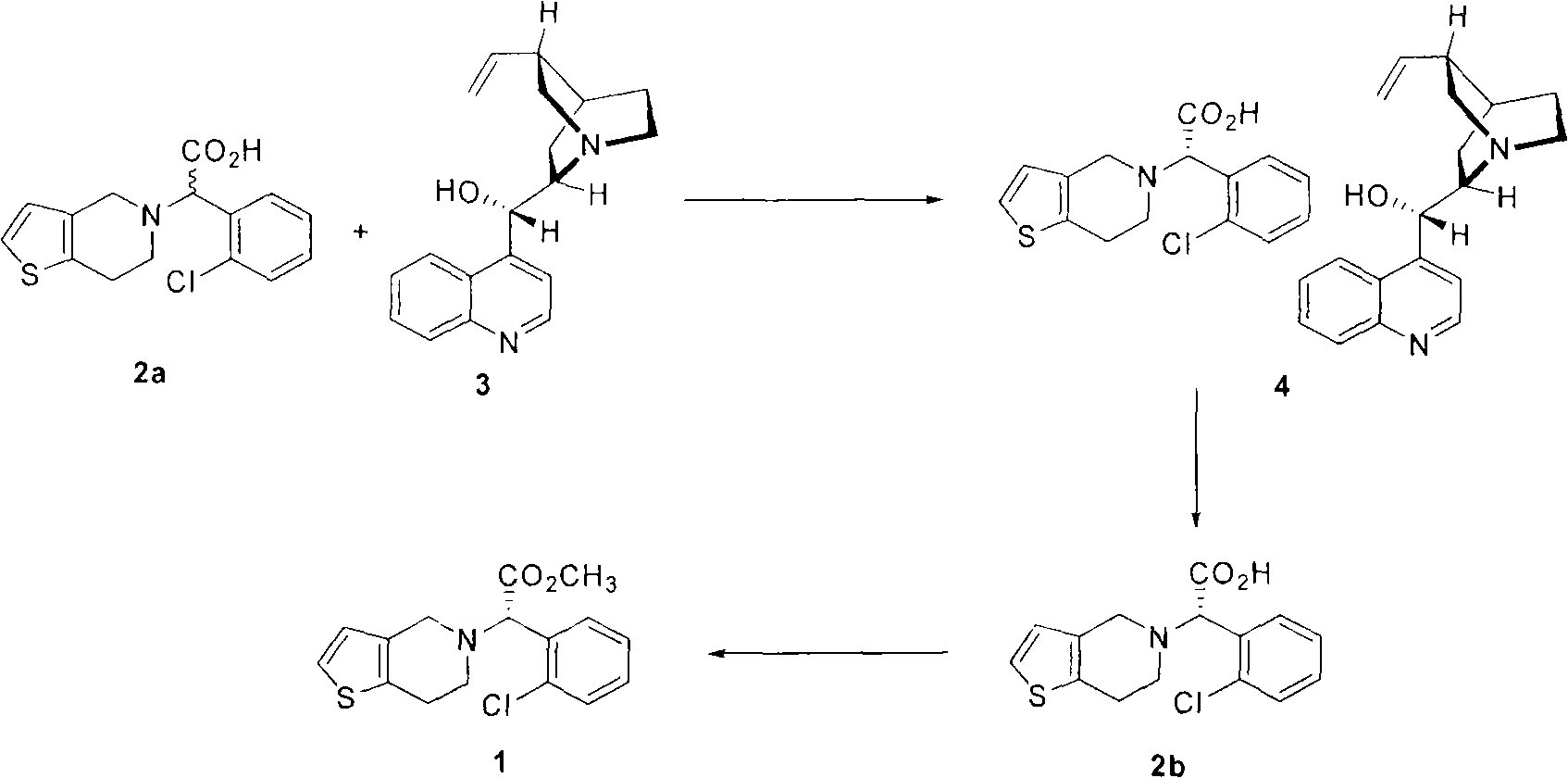
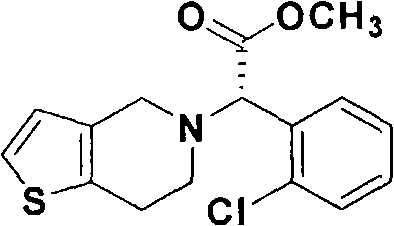
[0047] Example 1: Synthesis of diastereomeric salts (4) from the racemic carboxylic acid (2a) of clopidogrel
[0048] 3.078 grams (10 mmol) of the racemic carboxylic acid (2a) of clopidogrel and 3.47 grams (10 mmol) of 85% (+)-cinchonine were put into a 250 milliliter flask, and by adding 100 milliliters of ethanol thereto: acetonitrile (1 : 2) to dissolve it completely. After the resulting solution was shaken at room temperature for 18 hours, the formed precipitate was filtered under reduced pressure and then vacuum dried at room temperature to obtain 1.74 g of the diastereomeric salt (4) as a white solid.
[0049] Theoretical yield 58%; optical purity 98.9% (HPLC); 1 H NMR (300MHz, DMSO-d 6 )δ8.85(d, 1H, J=4.5Hz), 8.28(d, 1H, J=8.1Hz), 8.02(dd, 1H, J=8.1Hz, 1.2Hz), 7.24-7.76(m, 8H) , 6.74(d, 1H, J=5.1Hz), 6.01-6.13(m, 1H), 5.58(d, 1H, J=5.1Hz), 5.14(d, 1H, J=9.3Hz), 5.09(s , 1H), 4.64(s, 1H), 3.56-3.73(m, 2H), 3.25-3.32(m, 2H), 2.66-2.90(m, 7H), 2.28-2.34(m, 1H), 1.95-2....
Embodiment 2
[0050] Example 2: Synthesis of diastereomeric salts (4) from the racemic carboxylic acid (2a) of clopidogrel
[0051] 3.078 grams (10 mmol) of the racemic carboxylic acid (2a) of clopidogrel and 1.735 grams (5 mmol) of 85% (+)-cinchonine were put into a 250 milliliter flask, and by adding 100 milliliters of ethanol thereto: acetonitrile (1 : 2) to dissolve it completely. After shaking the resulting solution at room temperature for 18 hours, the precipitate thus formed was filtered under reduced pressure and then dried under vacuum at room temperature to obtain 2.42 g of the diastereomeric salt (4) as a white solid.
[0052] Theoretical yield 80%; optical purity 99.8% (HPLC)
Embodiment 3
[0053] Example 3: Synthesis of diastereomeric salts (4) from the racemic carboxylic acid (2a) of clopidogrel
[0054] The racemic carboxylic acid (2a) of 3.078 grams (10mmol) clopidogrel and 3.078 grams (10mmol) 85% (+)-cinchonine are put into 250 milliliters flasks, and by adding 100 milliliters of ethanols wherein: acetone (1 : 2) to dissolve it completely. After shaking the resulting solution at room temperature for 18 hours, the precipitate thus formed was filtered under reduced pressure and then dried under vacuum at room temperature to obtain 2.54 g of the diastereomeric salt (4) as a white solid.
[0055] Theoretical yield 84%; optical purity 99.8% (HPLC)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com