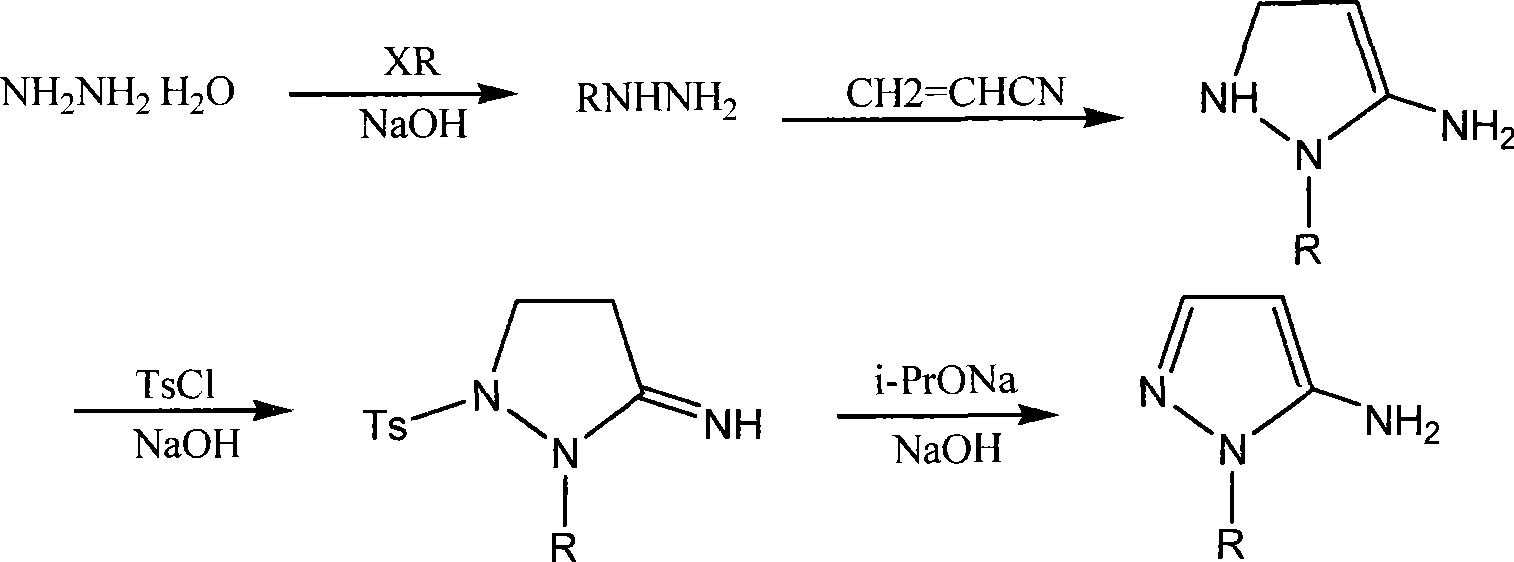
Novel synthesis process for 5-amino-1-hydroxyethyl pyrazole or the like
A technology for hydroxyethylpyrazole and its analogues, which is applied in the field of new synthesis technology of 5-amino-1-hydroxyethylpyrazole and its analogues, and can solve the problem of the high price of cefotaxime sulfate and the impact on market sales and popularization, high production costs and other issues, to achieve the effect of low cost, easy operation and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The synthesis of embodiment 1,5-amino-1-hydroxyethylpyrazole:
[0017] (1), the synthesis of β-hydroxyethylhydrazine
[0018] 32.0 grams of sodium hydroxide and 200.0 grams of 80% hydrazine hydrate were mixed together and stirred evenly, stirred at room temperature for 30 minutes, stirred at 70°C for 2.5 hours, cooled in an ice bath, added 64.4 grams of chloroethanol dropwise, and kept the temperature at 30 React at ~45°C for 2 hours, concentrate the reaction solution, remove solid sodium chloride by filtration, rinse the filter cake with ethyl acetate, and collect fractions at 127°C / 2.27KPa under reduced pressure. 1 H NMR: 2.64(t, 2H), 3.45(t, 2H), 3.67(br, 4H);
[0019] (2), the synthesis of 5-amino-1-hydroxyethyl-5-pyrazoline
[0020] Add dropwise a mixed solution of 53 g of acrylonitrile and 400 mL of toluene to 76.0 g of the above product, stir and react at 95-100°C for 8 hours, concentrate under reduced pressure, refrigerate to precipitate crystals, filter, rins...
Embodiment 2
[0025] The synthesis of embodiment 2,5-amino-1-hydroxyethylpyrazole analogue:
[0026] (1), the synthesis of β-hydroxypropylhydrazine
[0027] Mix 32.0 grams of sodium hydroxide and 200.0 grams of 80% hydrazine hydrate together and stir evenly, stir at room temperature for 30 minutes, stir at 70°C for 2.5 hours, cool in an ice bath, add 75.7 grams of chloropropanol dropwise, and after the dropwise addition, keep the temperature React at 40-55°C for 2.5 hours, concentrate the reaction solution, filter to remove solid sodium chloride, rinse the filter cake with ethyl acetate, and collect fractions under reduced pressure;
[0028] (2), the synthesis of 5-amino-1-hydroxypropyl-5-pyrazoline
[0029] Add dropwise a mixed solution of 53 g of acrylonitrile and 400 mL of toluene to 90.0 g of the above product, stir and react at 100-110°C for 8.5 hours, concentrate under reduced pressure, refrigerate to precipitate crystals, filter, rinse the filter cake with ice ethanol, and dry. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com