Chemical luminescence immune assay determination reagent kit for gastrin releasing peptide precursor
A gastrin-releasing peptide and chemiluminescence immunology technology, applied in the field of immunoassay medicine, can solve the problems of limited development and use, low analytical sensitivity, etc., and achieve the effects of reducing dosage, improving linear range and enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of the gastrin releasing peptide precursor (31-98) chemical method photoimmunoassay assay kit of the present invention
[0024] 1. Preparation of standard products
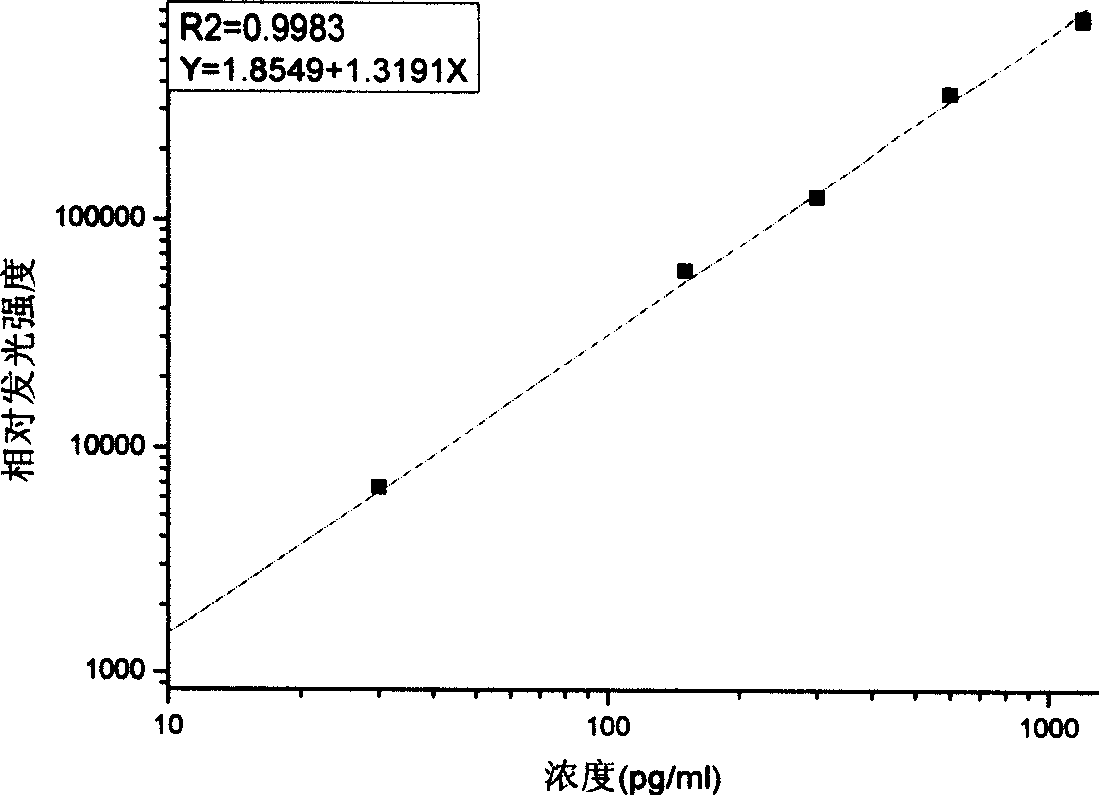
[0025] Select adult bovine serum as the standard matrix solution to dilute the pure product of proGRP (31-98), the concentrations are 0pg / ml, 30pg / ml, 150pg / ml, 300pg / ml, 600pg / ml, 1200pg / ml;
[0026] 2. Preparation of enzyme markers and determination of enzyme dilution concentration
[0027] 1. The improved glutaraldehyde cross-linking method marks horseradish peroxidase (HRP), and its steps are as follows:
[0028] a. Dissolve 12mg of HRP in 1ml of PBS (0.1Mol / L pH6.8) containing 5mg of proGRP monoclonal antibody, add 4mL of 1% glutaraldehyde solution under slow stirring, and leave at room temperature for 3 hours.
[0029] b. Add 0.1 mL of 0.2 mol / L lysine (0.29 g dissolved in 10 mL of distilled water), and place at 4°C for 2 hours to block the residual aldehyde groups and terminat...
Embodiment 2
[0070] Example 2 Method of using the gastrin releasing peptide precursor chemical method photoimmunoassay assay kit of the present invention
[0071] 1. Sample pretreatment
[0072] Take human fasting serum, centrifuge at 2000-4000rpm for 10min, and take the supernatant, which can be analyzed without any other special treatment.
[0073] 2. Detection steps
[0074] Before using this kit for detection, it is necessary to take out the components packaged in the kit first: the coated plate, enzyme markers, and standard products are allowed to stand at room temperature, and then used after equilibrating to room temperature; adjust the incubator or water bath to 37°C; Prepare a suitable micro-injector and corresponding tips and check whether the chemiluminescence instrument is working normally.
[0075] The specific operation steps for using this kit are as follows:
[0076] 1) Take out the kit and equilibrate to room temperature;
[0077] 2) Add 25 μL of serum sample or serial...
Embodiment 3
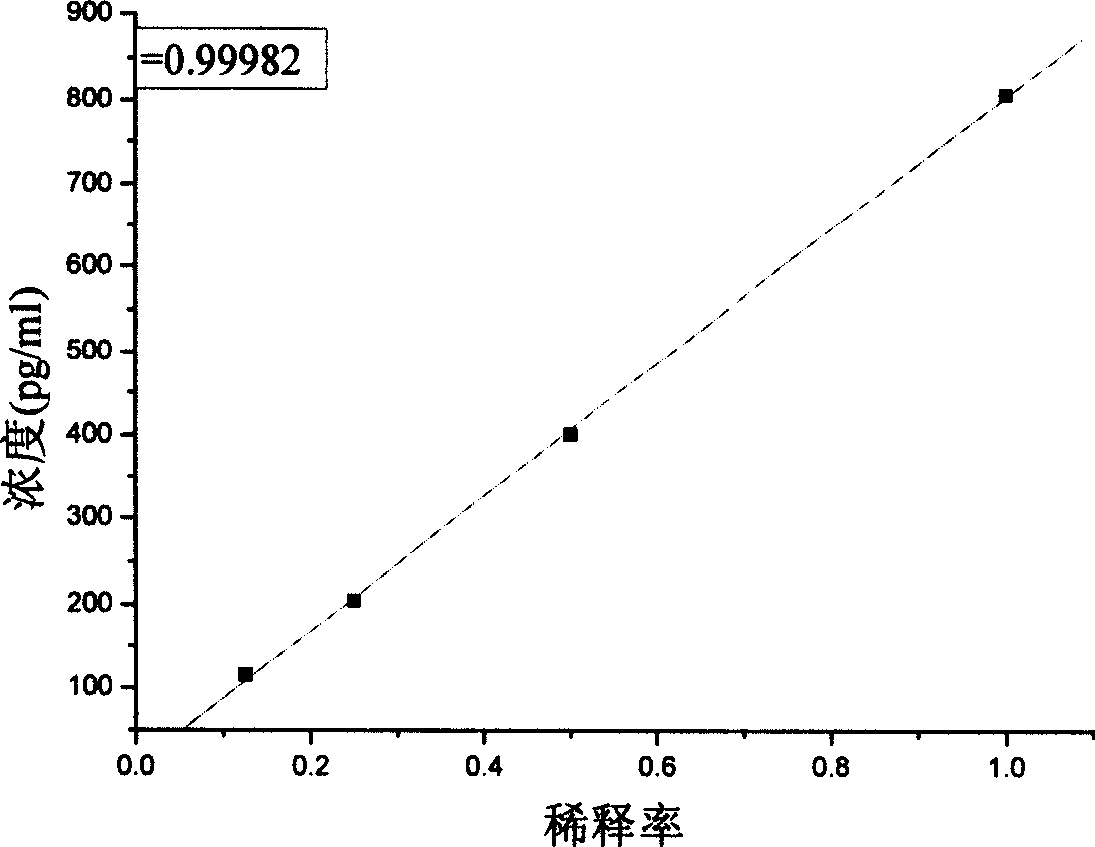
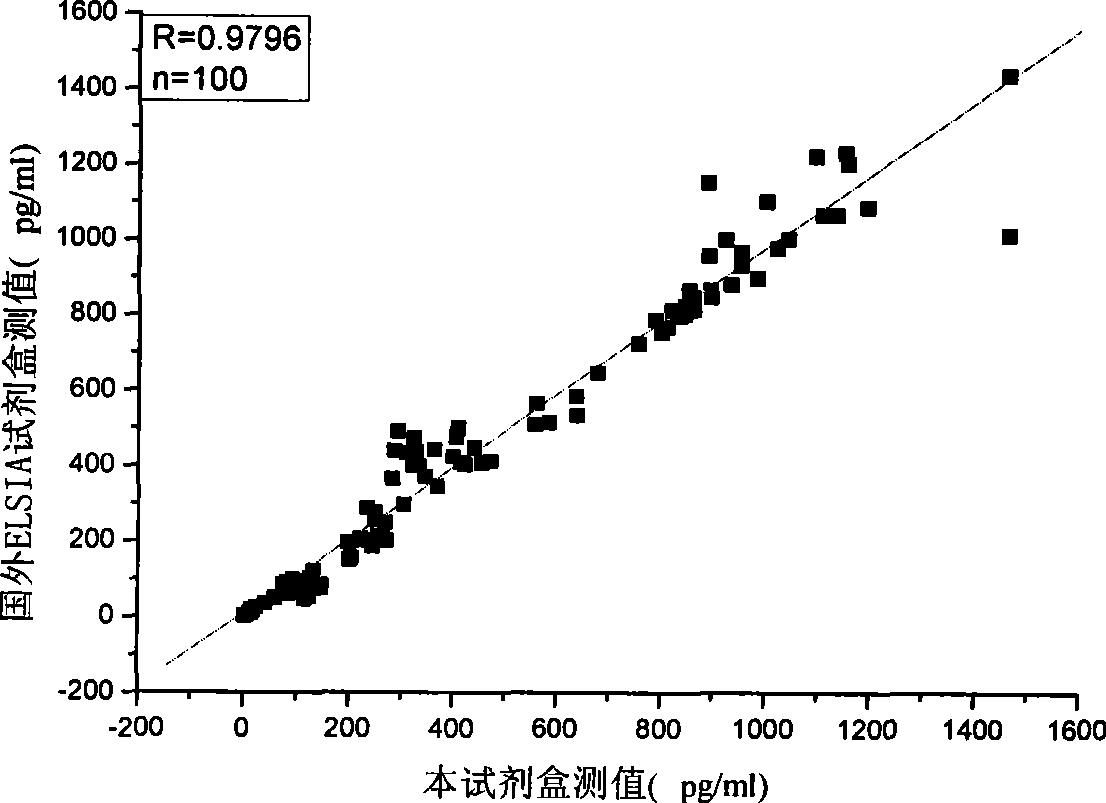
[0085] Embodiment 3 The methodological index of the kit of the present invention
[0086] The test kit prepared in Example 1 is tested according to the conventional manufacturing and testing procedures in the art, and the results are as follows:
[0087] 1. Kit sensitivity
[0088] Sensitivity is the concentration that is distinguishable from the zero dose point. Measure 10 zero standard points at the same time to obtain the average value and standard deviation of RLU. Calculate the difference between the average value of RLU and twice the standard deviation, and obtain the corresponding concentration value from the working curve by interpolation. The sensitivity determined in the experiment was 1.37pg / ml.
[0089] 2. Kit precision
[0090] Three batches of the kit prepared in Example 1 were taken respectively for precision experiment. With the kit extracted in Example 1, three high\middle\low value samples of 110pg / mL and 220pg / mL proGRP samples were measured 8 times eac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com