Compositions and methods for treating dermatological conditions
A preparation and skin technology, applied in the field of system to treat various skin symptoms, can solve problems such as drug retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
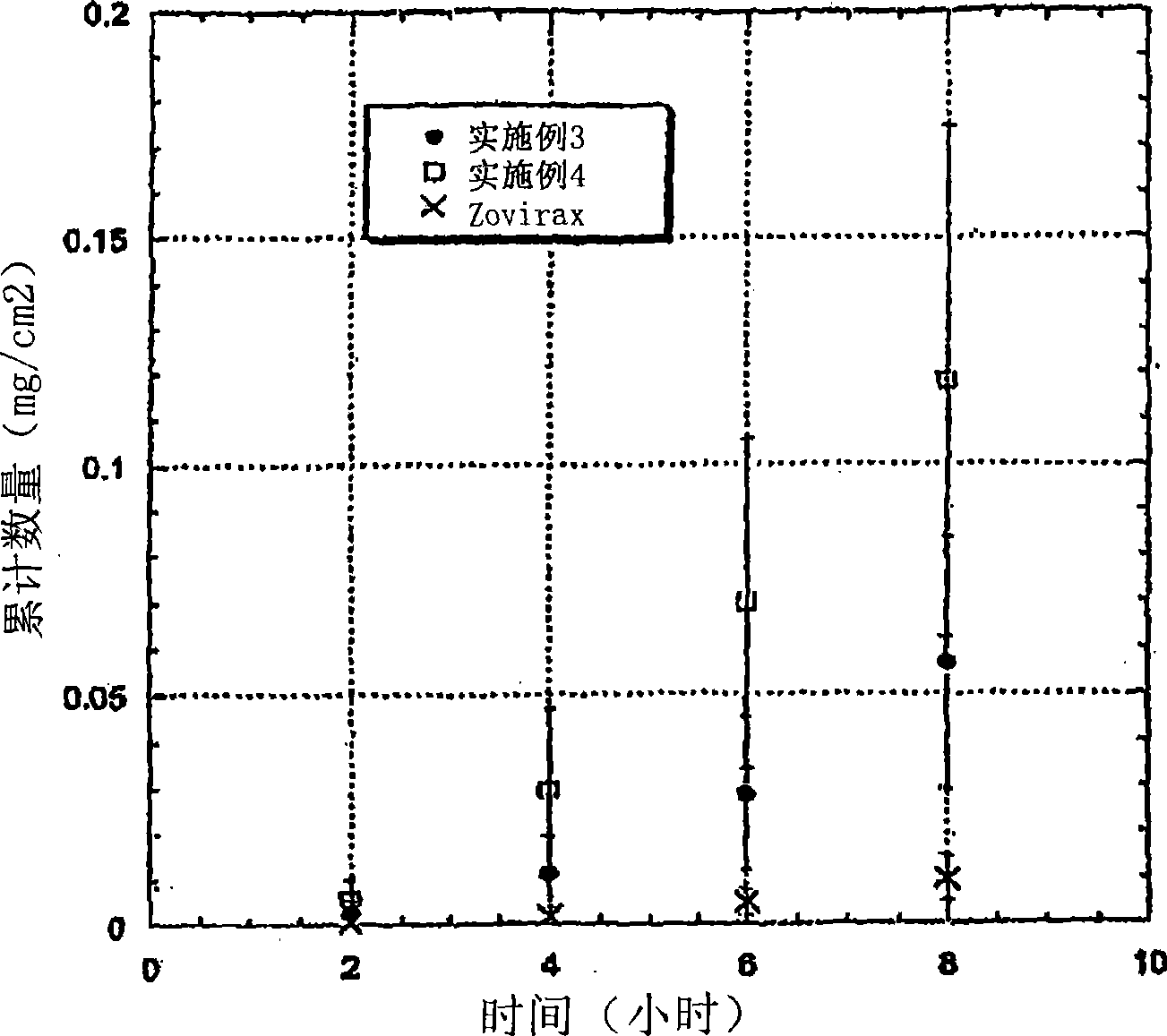
[0110] [0094] As indicated, hairless mouse skin (HMS) or human epidermal membrane (HEM) were used as model membranes for the in vitro flux studies described here. Hairless mouse skin (HMS) was used as a model membrane for the in vitro flux studies described here. Freshly isolated epidermis from the abdomen of hairless mice was carefully placed between the supply and recipient compartments of the Franz diffusion cell. The receptor chamber was filled with pH 7.4 phosphate buffered saline (PBS). The experiments were initiated by placing the test formulations (Examples 2-5) on the stratum corneum (SC) of the skin samples. The Franz cell was placed on a heating stage maintained at 37°C and the temperature of the HMS was maintained at 35°C. At preset time intervals, 800 μL aliquots were aspirated and replaced with fresh PBS solution. Skin flux (μg / cm 2 / h). It should be noted that human cadaver skin can also be used as a model membrane for in vitro flux studies. The skin was p...
Embodiment 2
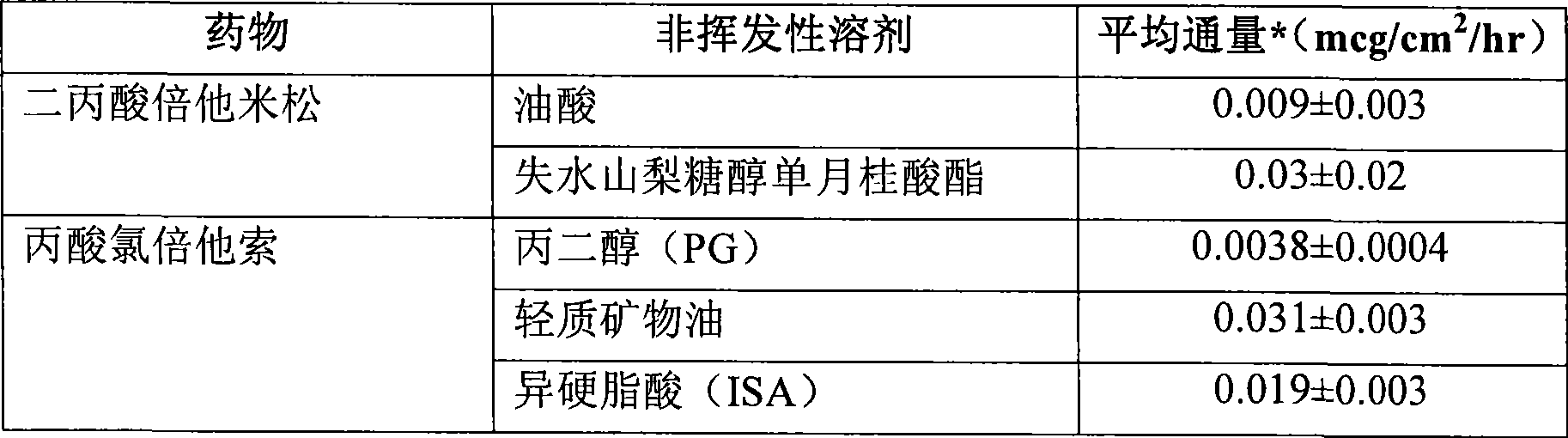
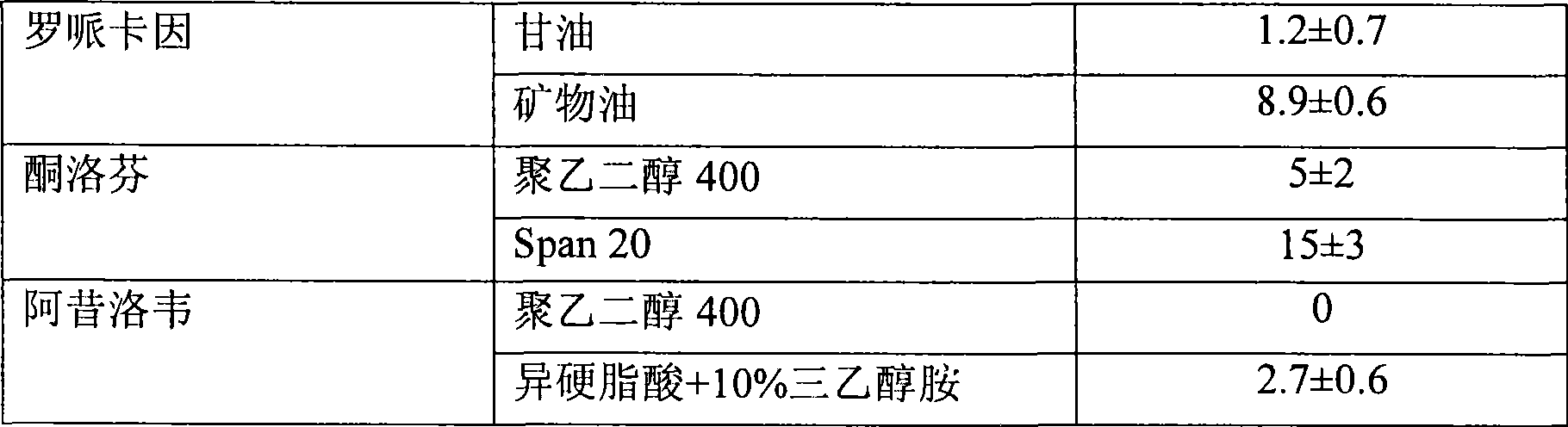
[0112] [0095] Acyclovir formulations in various non-volatile solvent systems were evaluated. There is an excess of aciclovir. The transdermal flux of acyclovir from the test formulations by HMS is shown in Table 1 below.
[0113] Table 1
[0114] non-volatile solvent system
skin flux *
(mcg / cm 2 / h) Isostearic acid 0.1±0.09 Isostearic Acid + 10% Triethanolamine 2.7±0.6 Isostearic Acid + 30% Triethanolamine 7±2 olive oil 0.3±0.2 Olive Oil + 11% Triethanolamine 3±3 Olive Oil + 30% Triethanolamine 0.3±0.2 Oleic acid 0.4±0.3 Oleic Acid + 10% Triethanolamine 3.7±0.5 Oleic Acid + 30% Triethanolamine 14±5 ethyl oleate 0.2±0.2 Ethyl Oleate + 10% Triethanolamine 0.2±0.2
[0115] * Skin flux measurements represent the mean and standard deviation of three determinations. Reported flux measurements were determined from the linear region of the cumulant versus time curve. A linear region is obser...
Embodiment 3-6
[0118] [0097] Prototype viscous curing formulations were prepared as follows. According to Table 2, prepare some acyclovir solidified preparations according to the embodiment of the present invention, as follows:
[0119] Table 2
[0120]
[0121]
[0122] * Degussa polymer
[0123][0098] In Examples 3-6, the compositions in Table 2 were prepared as follows. Eudragit RL-PO and ethanol were mixed in a glass jar and heated with stirring until the RL-PO dissolved. Isostearic acid and triethanolamine were added to the RL-PO / ethanol mixture and the mixture was stirred vigorously. Once a homogeneous mixture is obtained, acyclovir is added to the mixture and the formulation is mixed vigorously.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com