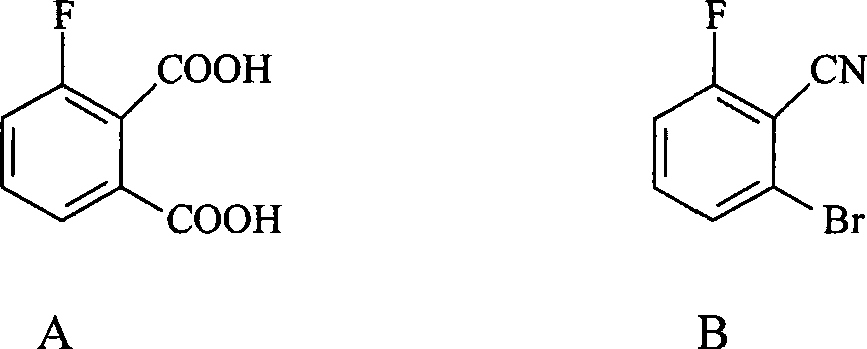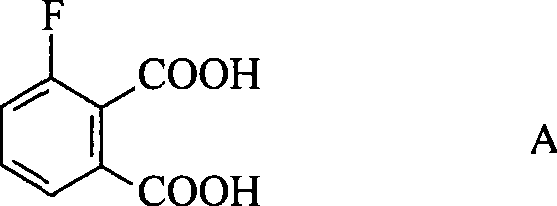Preparation method of 3-fluorophthalic acid
A technology of fluorophthalonitrile and fluorobenzonitrile, which is applied in the field of preparing 3-fluorophthalic acid, and can solve the problems of low yield of target product, low purity of target product, and difficult preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] The method for preparing 3-fluorophthalic acid (structure shown in formula A) of the present invention comprises the following steps:
[0016] 1. With 2,6-difluorobenzonitrile as starting raw material, in aprotic solvent, 2,6-difluorobenzonitrile is carried out ammoniation reaction (reaction with ammonia gas), reacts at least four hours, obtains 2- Amino, 6-fluorobenzonitrile.
[0017] Wherein: the reaction temperature of said ammoniation reaction should be set to 100 DEG C~130 DEG C (100 DEG C~110 DEG C of more preferred reaction temperature); Said aprotic solvent can be selected from sulfolane, dimethyl sulfoxide, N, One, two or more mixtures of N-dimethylacetamide or N,N-dimethylformamide; the amount of said aprotic solvent is the starting material (2,6-difluorobenzonitrile) 2 to 14 times the weight.
[0018] II. First carry out the diazotization reaction of 2-amino and 6-fluorobenzonitrile, that is, under the condition of 50°C to 70°C (the best temperature is 60°C...
Embodiment
[0026] I. Ammonification:
[0027] Add 200g (1.439mol) 2,6-difluorobenzonitrile and 600mL dimethyl sulfoxide into a 5L three-necked flask equipped with a spherical condenser, a thermometer, and an electric stirrer, and pass ammonia gas at room temperature for 30 minutes (pass ammonia gas at room temperature When the reaction bottle was a milky white turbid liquid), the temperature was raised to 100 ° C ~ 110 ° C under the condition of continuing to pass the ammonia gas, and the reaction was stopped after 4 hours. The dripped liquid was transparent, and then about 1400-1500 mL of water was added to the reaction bottle, the solid was precipitated by cooling, filtered, and washed with water to obtain 140 g of 2-amino-6-fluoro-benzonitrile, yield: 71.5%, content: 98%. Melting point: 128~130℃.
[0028] 1 H NMR (acetone-d 6 )δ (ppm): 5.82 (br s, 2H, NH 2 ); 6.35-7.48 (m, 3H, Ph).
[0029] 19 F NMR (CD 3 SOCD 3 , CFCl 3 ), δ (ppm): 108.8.
[0030] II. Diazotization and brom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com