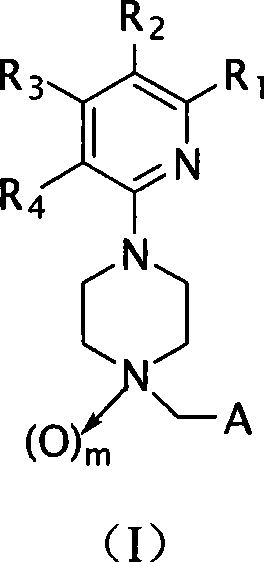
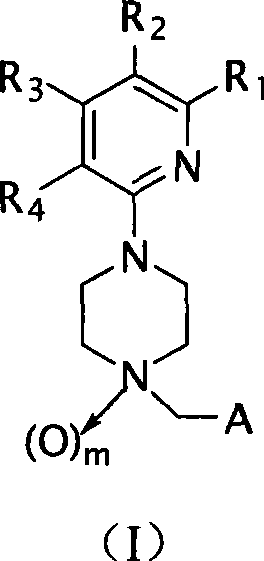
Piperazine derivatives and applications thereof
A derivative, piperazine technology, applied in the field of piperazine derivatives, can solve problems such as ligand differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
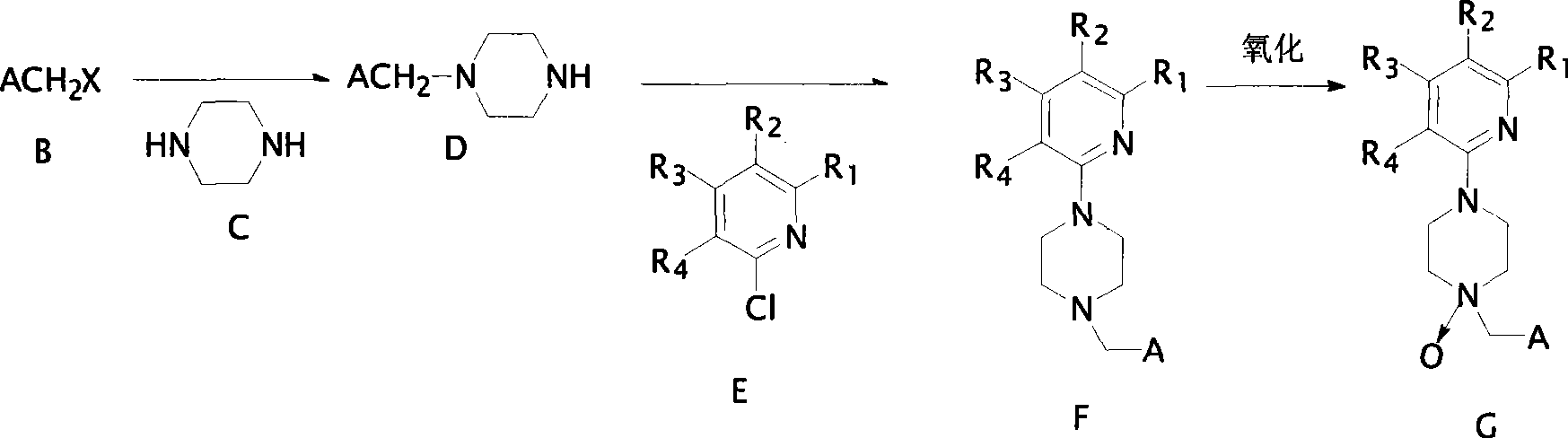
Image
Examples
Embodiment 1
[0026] Synthesis of 1-(6-chloropyridine-3-methyl)-4-(5-trifluoromethylpyridine-2-)piperazine
[0027] (1) Synthesis of 1-(6-chloropyridine-3-methyl)piperazine:
[0028] The reaction equation is as follows:
[0029]
[0030] A solution of 1.61g (10mmol) of 6-chloro-3-chloromethylpyridine in 10ml of absolute ethanol was slowly added dropwise in an oil bath at 50-55°C to dissolve 1.32g (15mmol) of anhydrous piperazine and 10mmol of tris In 15ml of absolute ethanol solution of ethylamine, the system was stirred for 5-6 hours, and the raw material 6-chloro-3-chloromethylpyridine was completely reacted. The solvent ethanol was evaporated to obtain the crude product, which was washed with water, extracted with dichloromethane and anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a pure product with a yield of about 86%.
[0031] GC / MS(m / s)211(38)M + , 169(100), 126(78), 56(23).
[0032] (2) Synthesis of 1-(6-chloropyridine-3-methyl...
Embodiment 2
[0039] Synthesis of 1-(6-chloropyridine-3-methyl)-4-(5-trifluoromethylpyridine-2-)piperazine peroxide,
[0040] The reaction equation is as follows:
[0041]
[0042] In an ice bath, to a solution of 1mmol 1-(6-chloro-pyridine-3-methyl)-4-(5-trifluoromethylpyridine-2-)piperazine in 20ml of dichloromethane solution was added twice 1 mmol m-chloroperoxybenzoic acid (MCPBA). Then, the reaction system was stirred at room temperature, followed by TLC, and after 12 hours of reaction, the reaction of raw materials was complete. The reaction mixture was poured into water, extracted three times with dichloromethane, the organic phases were combined, washed with saturated brine and water, and dried over anhydrous sodium sulfate. After evaporating the solvent under reduced pressure, the crude product was obtained. The product was separated by column chromatography, eluting with dichloromethane / ethanol (v / v=8:1), and the solvent was evaporated under reduced pressure to obtain a red s...
Embodiment 3
[0046] Synthesis of 1-(2-chlorothiazole-5-methyl)-4-(3-chloro-5-trifluoromethylpyridine-2-)piperazine
[0047] (1) Synthesis of 1-(2-chlorothiazole-2-methyl)piperazine
[0048] The reaction equation is as follows:
[0049]
[0050] A solution of 1.67g (10mmol) of 2-chloro-5-chloromethylthiazole in 10ml of absolute ethanol was slowly added dropwise in an oil bath at 50-55°C to dissolve 1.32g (15mmol) of anhydrous piperazine and 10mmol of tris In 15ml of absolute ethanol solution of ethylamine, the system was stirred for 5-6 hours, and 1.67g (10mmol) of the raw material 2-chloro-5-chloromethylthiazole was completely reacted. The solvent ethanol was evaporated to obtain the crude product, which was washed with water, extracted with dichloromethane and anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a pure product with a yield of about 87%. GC / MS(m / s)217(44)M + , 175(79), 132(100), 56(31).
[0051] (2) Synthesis of 1-(2-chloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com