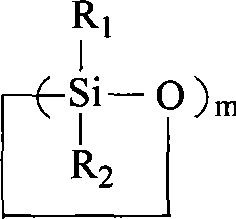Polysiloxane binary alcohol with polyether block and preparation method thereof
A technology of polysiloxane and polyether blocks, which is applied in the field of polymer material intermediate polysiloxane diol and its preparation, can solve the problems of low reactivity, hydrolysis resistance and poor storage stability, and achieve good Storage stability, improvement of low reactivity, wide range of application effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 149.0g octamethylcyclotetrasiloxane (D 4 ) and 13.4g of tetramethyldisiloxane were added to a dry 500mL three-necked flask equipped with a thermometer, condenser and stirring, and then 1.62g of concentrated sulfuric acid was added to react at 45°C for 48h; the product was washed with deionized water , Distilled under reduced pressure to obtain 146 g of linear dihydrogen-terminated polysiloxane (HPDMS).
[0032] Add the above 146g HPDMS into a 500mL three-necked flask, and then add 80.5g monoallyl-terminated polyethylene oxide (MAPEO, M n =350), when the temperature was raised to 80°C, 0.65 mL of chloroplatinic acid solution was added, and the temperature was continued to rise to 100°C and reacted at 100°C for 5 h. After the unreacted MAPEO and catalyst were washed away with deionized water, 216 g of polyether block polysiloxane diols were obtained by distillation under reduced pressure.
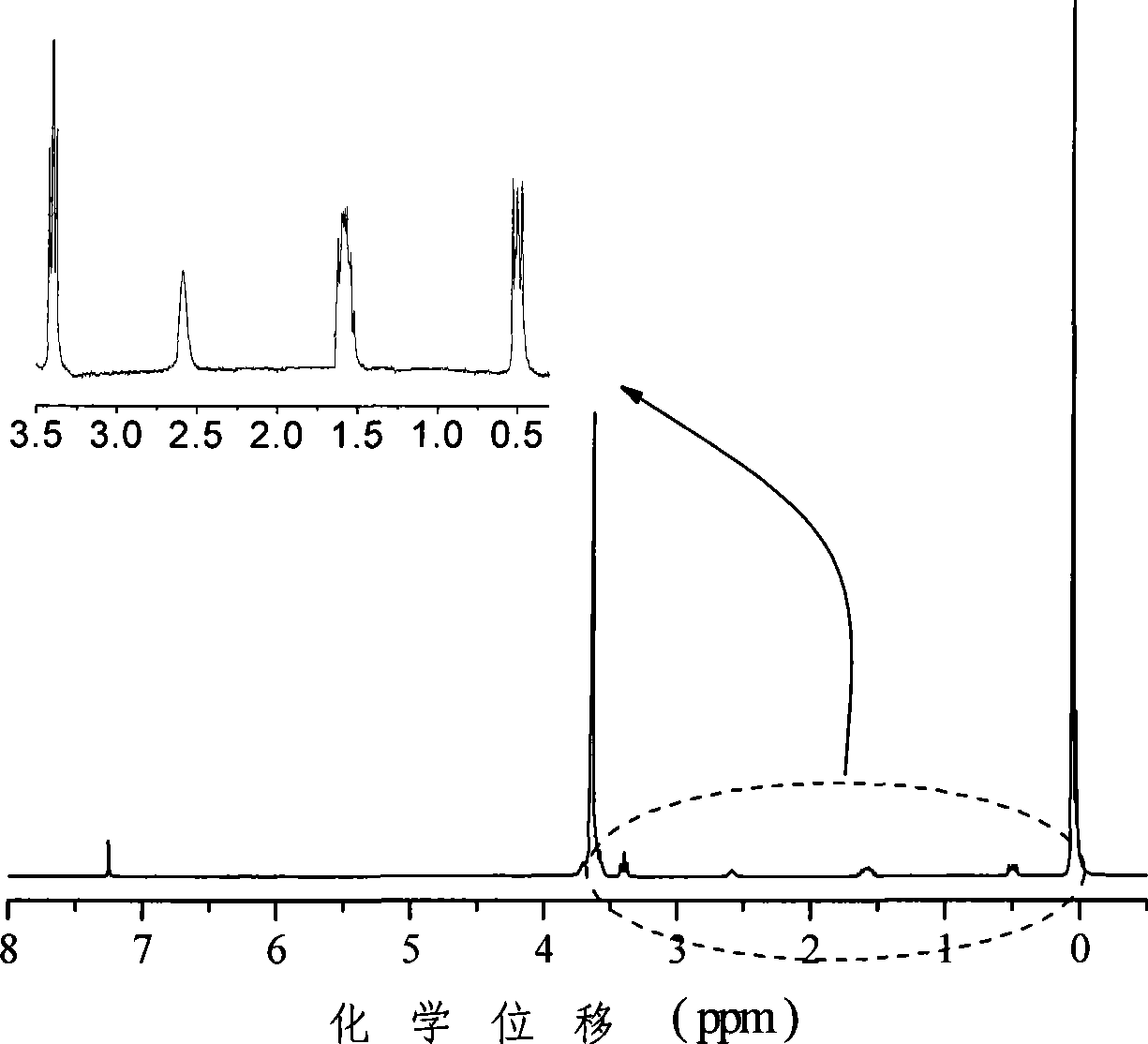
[0033] attached figure 1 The final product of this embodiment is given 1 H nuc...
Embodiment 2
[0037] 238.4g D 4 Add 13.4g of tetramethyldisiloxane into a dry 500mL three-necked flask equipped with a thermometer, condenser and stirring, then add 1.26g of trifluoroacetic acid, react at 45°C for 72h; wash the product with deionized water , Distilled under reduced pressure to obtain 213g HPDMS.
[0038]Add above-mentioned 213g HPDMS in 500mL there-necked bottle, then add 80.5g MAPEO (M n =350), when the temperature was raised to 80°C, 0.89 mL of chloroplatinic acid solution was added, and the temperature was continued to rise to 100°C and reacted at 100°C for 5 h. After the unreacted MAPEO and the catalyst were washed away with deionized water, 281 g of polyether block polysiloxane diols were obtained by distillation under reduced pressure.
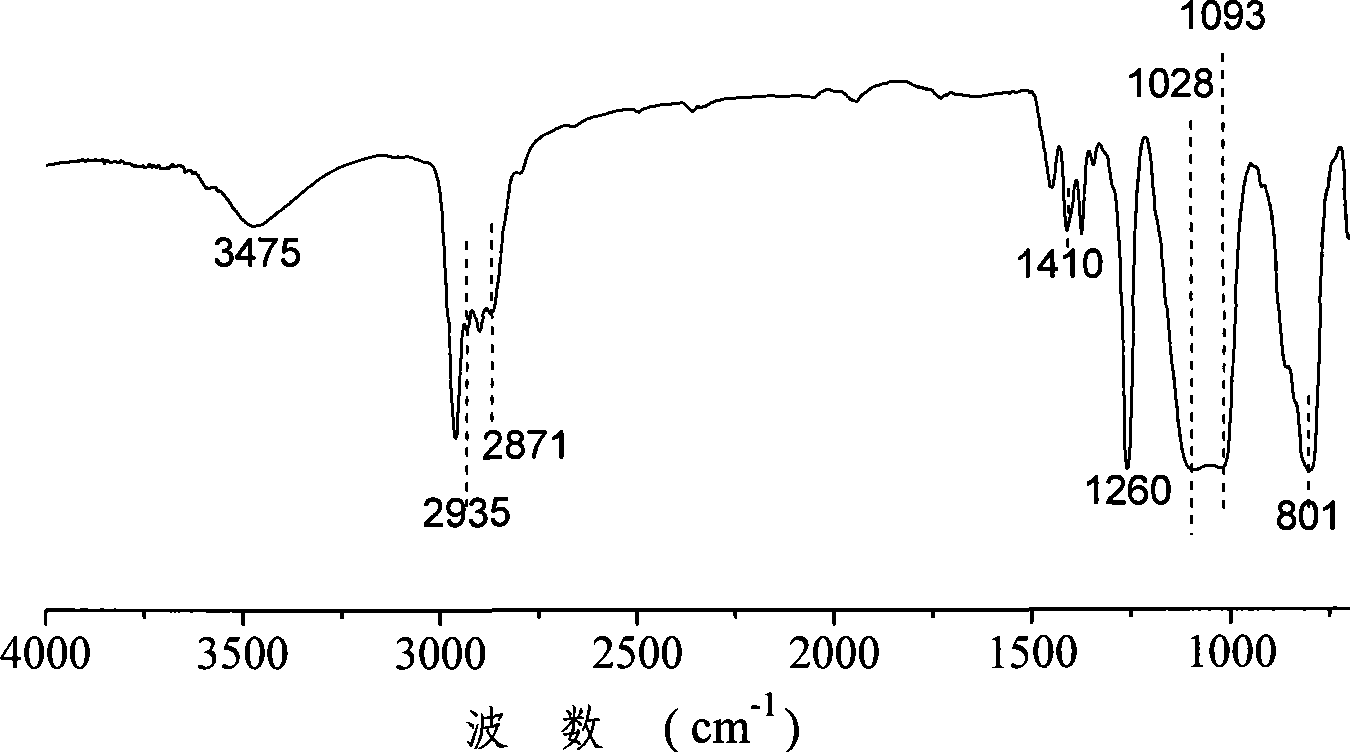
[0039] The infrared spectrogram of the resulting final product is attached figure 2 .
Embodiment 3
[0041] 112g hexamethylcyclotrisiloxane (D 3 ) and 13.4g of tetramethyldisiloxane were added to a dry 500mL three-necked flask equipped with a thermometer, condenser and stirring, and then 1.3g of concentrated sulfuric acid was added to react at 45°C for 48h; the product was washed with deionized water , Distilled under reduced pressure to obtain 118g HPDMS.
[0042] Add above-mentioned 118g HPDMS in 500mL there-necked bottle, then add 80.5gMAPEO (M n =350), when the temperature was raised to 80°C, 0.65 mL of chloroplatinic acid solution was added, and the temperature was continued to rise to 100°C and reacted at 100°C for 5 h. After the unreacted MAPEO and the catalyst were washed away with deionized water, 195 g of polyether block polysiloxane diol was obtained by distillation under reduced pressure.
[0043] The infrared spectrogram of the resulting final product is attached figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com