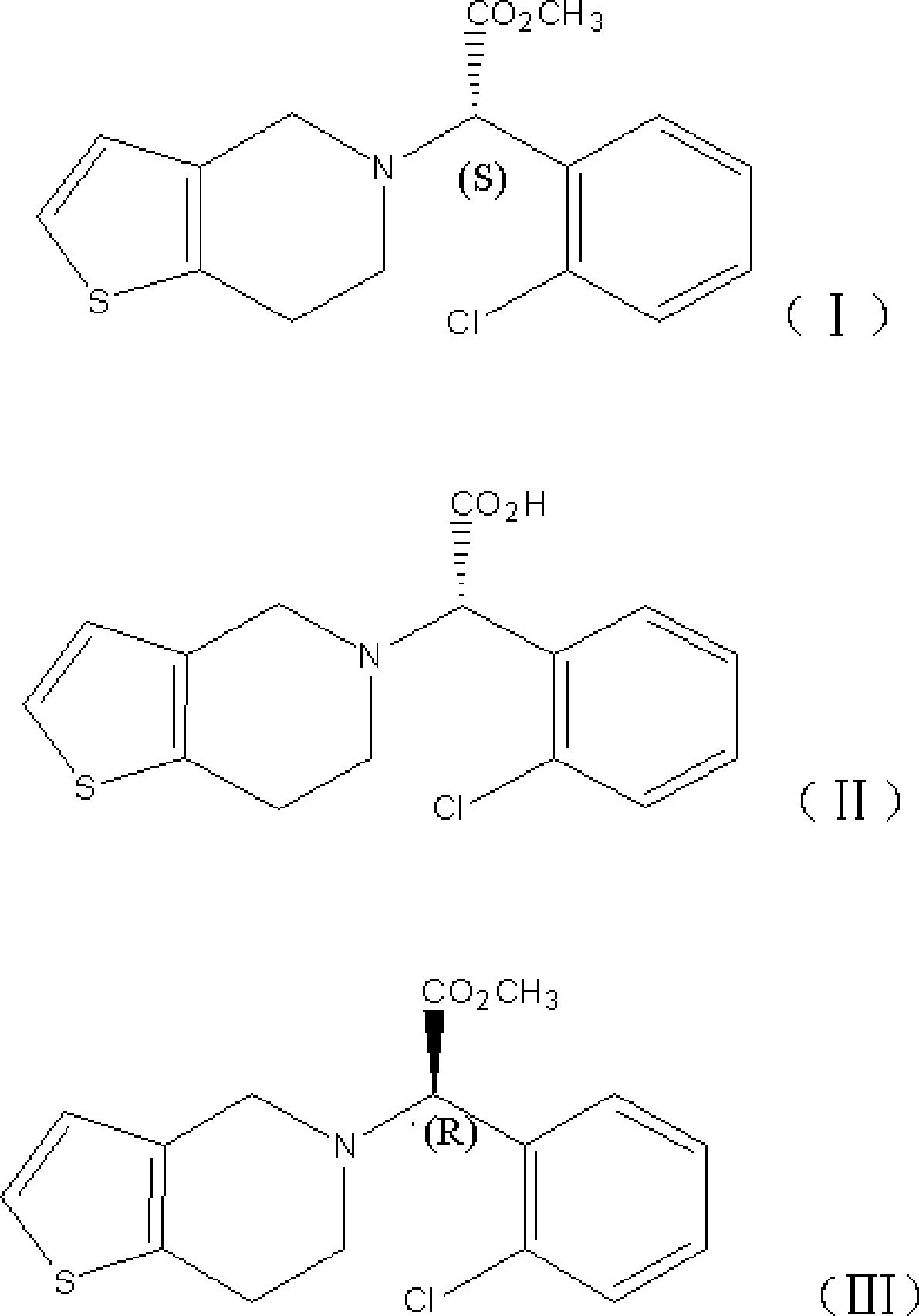
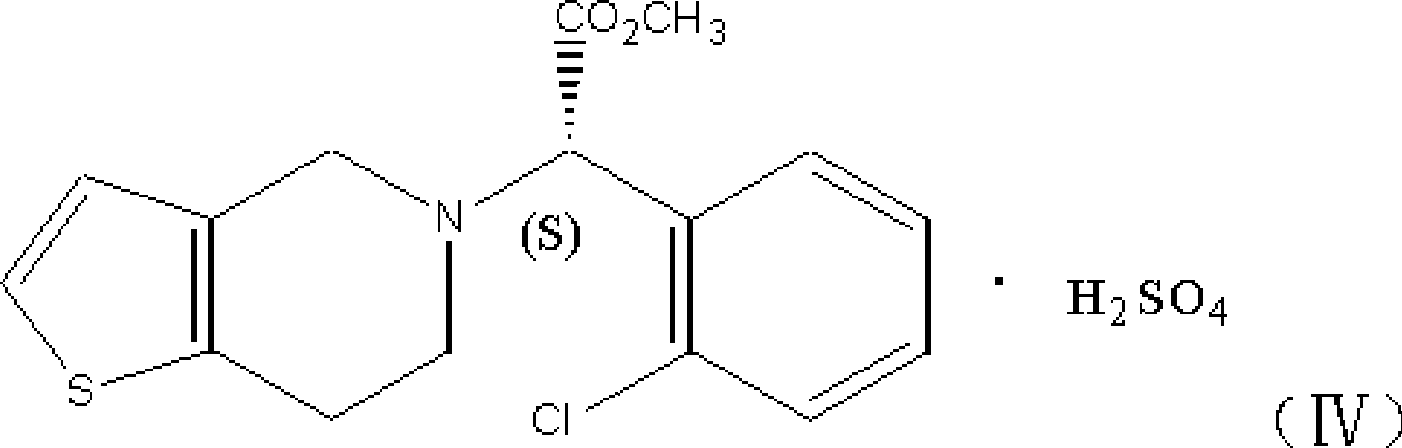
Solid preparation of clopidogrel and preparation method thereof
A clopidogrel, solid preparation technology, applied in the field of medicine, can solve the problem of no anti-platelet aggregation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
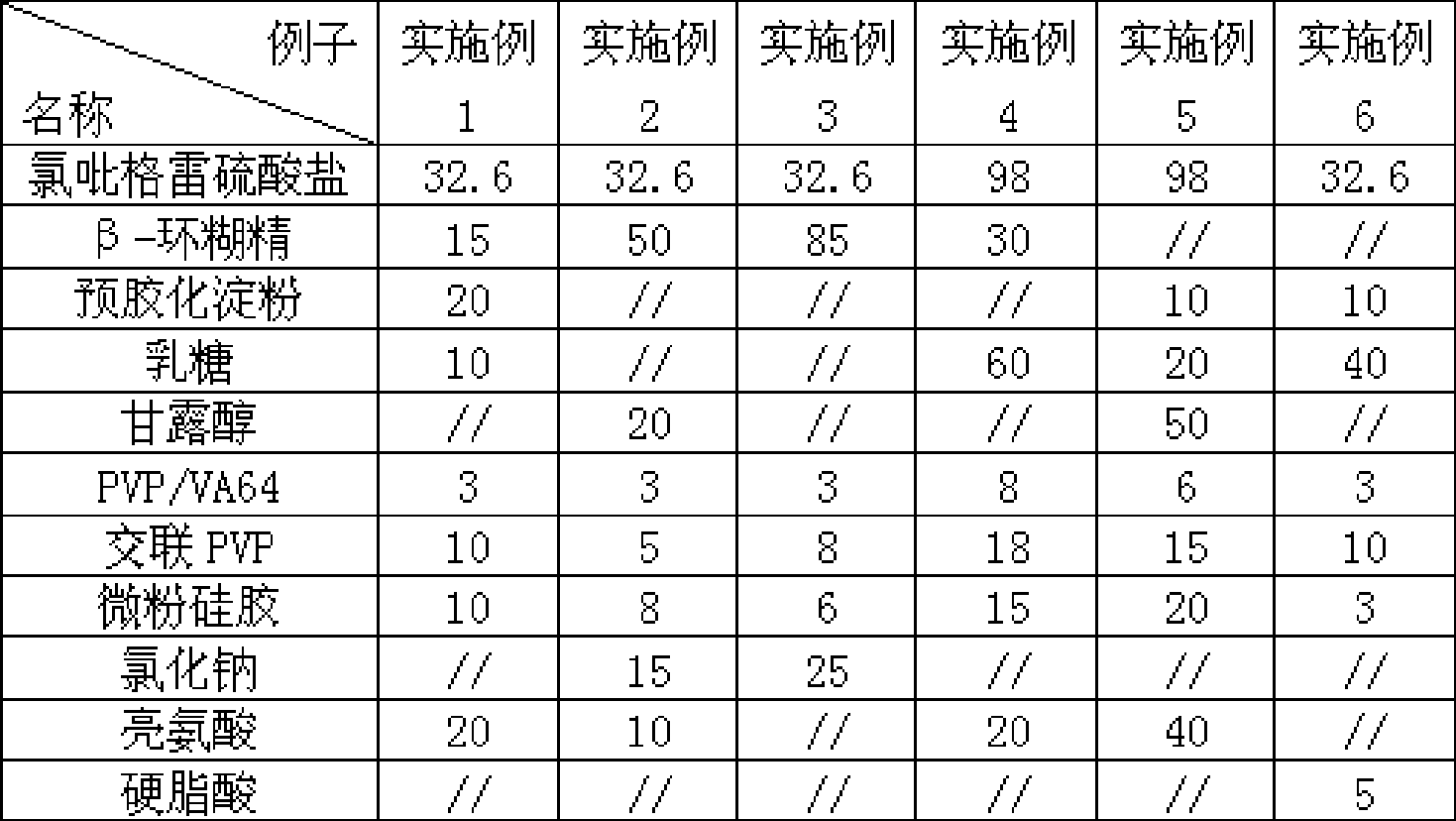
[0039] The preparation method of embodiment 1:
[0040] 1) Crush clopidogrel sulfate and β-cyclodextrin and sieve through a 120-mesh sieve, then mix it with pregelatinized starch, micropowder silica gel and anhydrous lactose, and press it into tablets. After crushing the obtained tablet, pass through a 30-mesh sieve to granulate;
[0041] 2) Mix the granules obtained in operation 1) with polyvinylpyrrolidone copolymer VA64, cross-linked polyvinylpyrrolidone and leucine evenly, and then directly compress to obtain tablets or fill capsule shells or directly seal them into aluminum-plastic composite film bags;
[0042] 3) The obtained plain tablet is coated with a film coating with Opadry II coating powder, the coating temperature is 50° C., and the weight gain after coating is 2.3%.
Embodiment 2
[0043] The preparation method of embodiment 2:
[0044] 1) Mix clopidogrel sulfate and β-cyclodextrin through a 100-mesh sieve, polyvinylpyrrolidone copolymer VA64, micropowder silica gel, cross-linked polyvinylpyrrolidone, sodium chloride and leucine, and then press directly Get tablets or fill capsule shells or directly seal them in aluminum-plastic composite film bags;
[0045] 2) The obtained plain tablet is coated with a film coating with Opadry II coating powder, and the weight gain is controlled to 2.5% after coating.
Embodiment 3
[0046] The preparation method of embodiment 3:
[0047] 1) After crushing clopidogrel sulfate and β-cyclodextrin through a 100-mesh sieve, mix it evenly with polyvinylpyrrolidone copolymer VA64, micropowder silica gel, cross-linked polyvinylpyrrolidone, and sodium chloride, and then directly compress to obtain tablets or Filling capsule shells or directly sealing them into aluminum-plastic composite film bags;
[0048] 2) The obtained plain tablet is coated with a film coating with Opadry II coating powder, and the weight gain is controlled to 2.5% after coating.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com