Method for producing isooctene with coproduction of sec-butyl carboxylate with selective superposition of butylene
A technology of acid sec-butyl ester and isooctene is applied in the field of selective lamination of butene to produce isooctene and co-production of sec-butyl carboxylate. Low and other problems to achieve the effect of prolonging life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
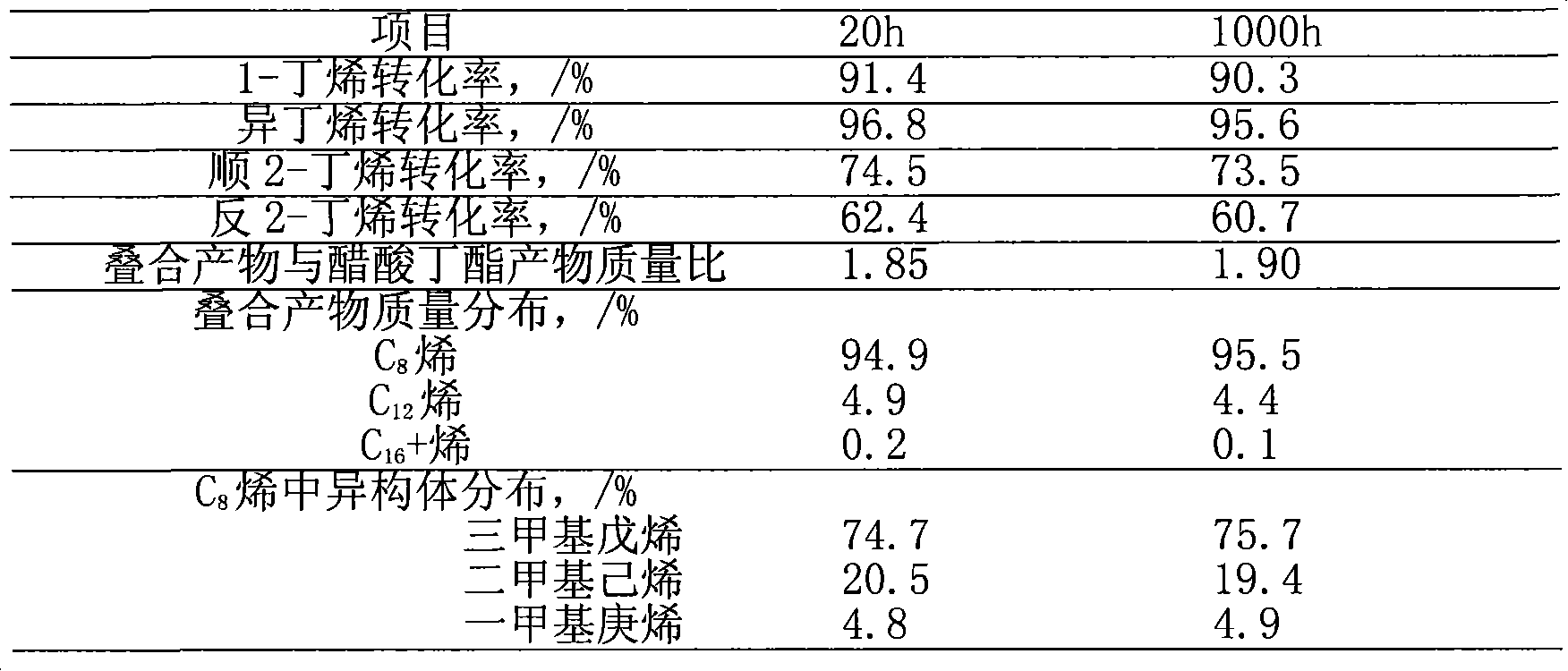
[0028] This example supports cesium phosphotungstate salt (Cs) with silica 2.5 h 0.5 PW 12 o 40 / SiO 2 ) as a catalyst, under the condition of adding acetic acid (acid-ene molar ratio is 0.2), the mixture C 4 to fold. Raw material C4 mass composition: isobutane 31.2%, n-butane 12.7%, n-butene 14.5%, isobutene 16.8%, trans-2-butene 15.0%, cis-2-butene 9.6%, 1,3-butadiene 0.2%.
[0029] The superposition reaction was carried out on a laboratory fixed-bed reaction device. The reactor is a stainless steel pipe with a circulating water jacket outside of φ20×4×600 mm. C 4 The raw material and acetic acid enter the reactor from the lower part of the reactor through the metering pump respectively from the raw material storage tank. The reaction product discharged from the top of the reactor first passes through the six-way valve, then passes through the back pressure valve, the condenser, and finally is collected in the product storage tank. The reaction temperature is contr...
example 2
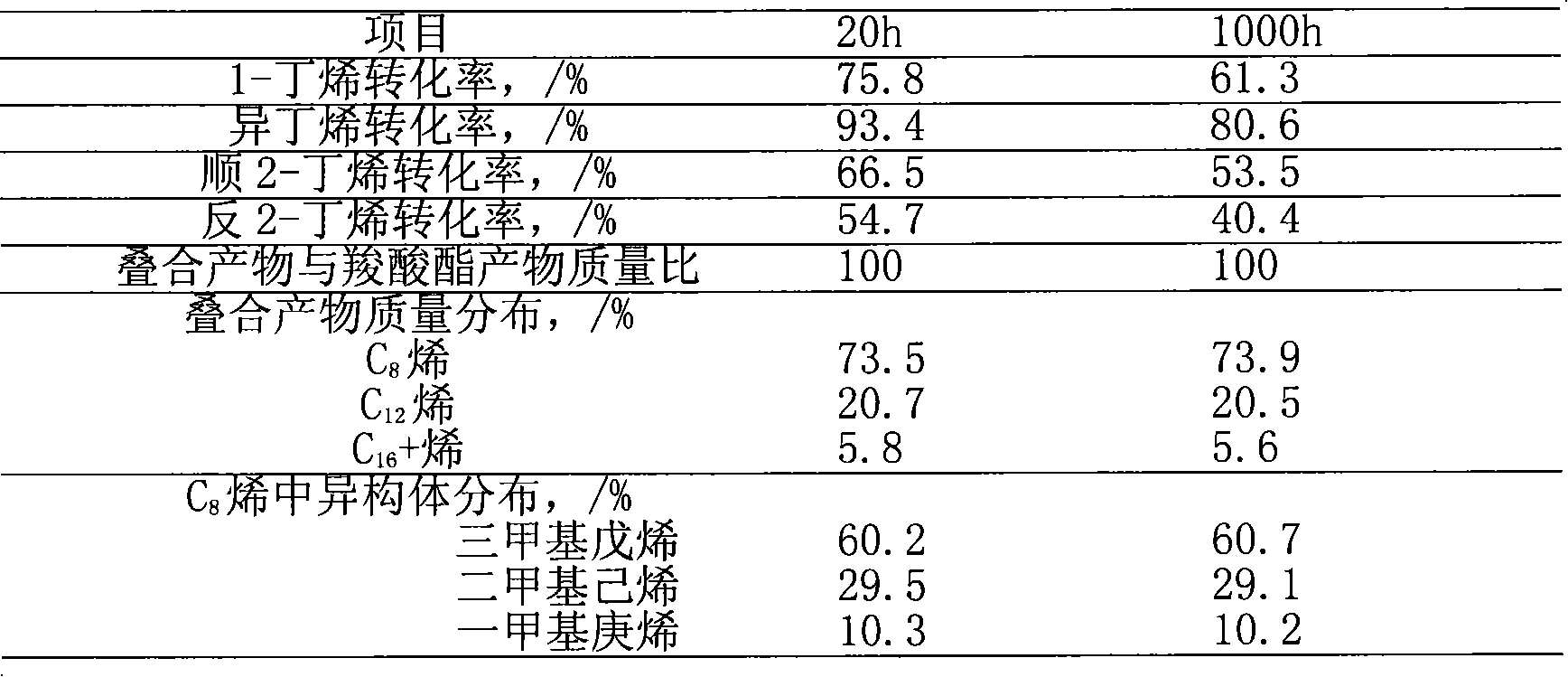
[0039] This example supports cesium phosphotungstate salt (Cs) with silica 2.5 h 0.5 PW 12 o 40 / SiO 2 ) is a catalyst, under the condition that acetic acid exists (the molar ratio of acetic acid and butene is 0.5), will mix C 4 to fold. raw material C 4 Mass composition: isobutane 31.2%, n-butane 12.7%, n-butene 14.5%, isobutene 16.8%, trans-2-butene 15.0%, cis-2-butene 9.6%, 1,3-butadiene 0.2% .
[0040] 20g Cs 2.5 h 0.5 PW 12 o 40 / SiO 2 Charge to the reactor of the reaction system described in Example 1. mix C 4 At 40g / h, acetic acid is continuously fed into the reaction system at a rate of 12g / h. The process conditions for controlling the reaction are: acid-ene molar ratio 0.5, reaction temperature 80°C, pressure 1.5MPa, for C 4 Feed weight hourly space velocity 2.0h -1 . After the reaction status is stable, the online sampling and analysis through the six-way valve is carried out regularly. The reaction was carried out continuously for 1000h. Table 3 is...
example 3
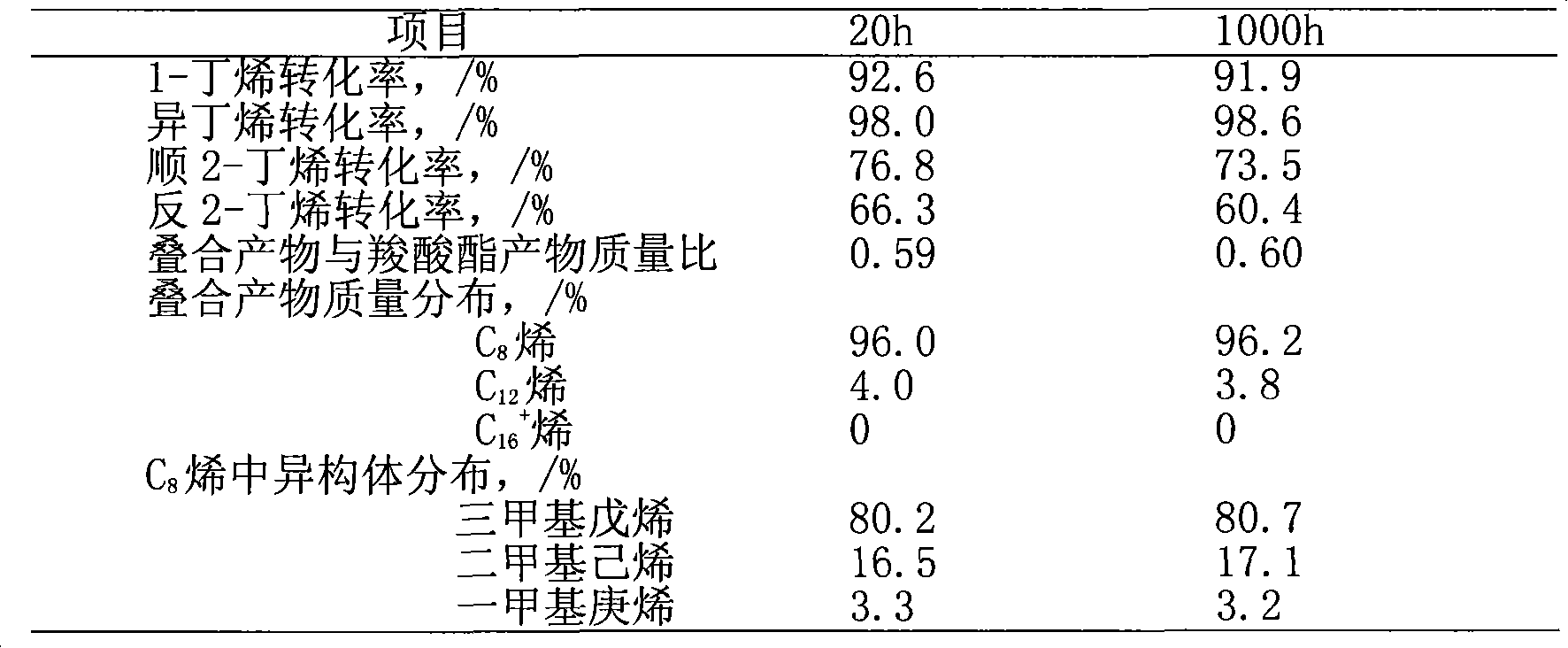
[0044] This example supports cesium phosphotungstate salt (Cs) with silica 2.5 h 0.5 PW 12 o 40 / SiO 2 ) as a catalyst, under the condition of adding propionic acid (acid-ene molar ratio is 0.2), the mixture C 4 to fold. Raw material C4 mass composition: isobutane 31.2%, n-butane 12.7%, n-butene 14.5%, isobutene 16.8%, trans-2-butene 15.0%, cis-2-butene 9.6%, 1,3-butadiene 0.2%.
[0045] 20g Cs 2.5 h 0.5 PW 12 o 40 / SiO 2 Charge to the reactor of the reaction system described in Example 1. mix C 4 At 40g / h, propionic acid is continuously fed into the reaction system at a rate of 6.0g / h. The process conditions for controlling the reaction are: acid-ene molar ratio 0.2, reaction temperature 80°C, pressure 1.5MPa, for C 4 Feed weight space velocity 2.0h -1 . After the reaction status is stable, the online sampling and analysis through the six-way valve is carried out regularly. The reaction was carried out continuously for 1000h. Table 4 is the conversion rate an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com