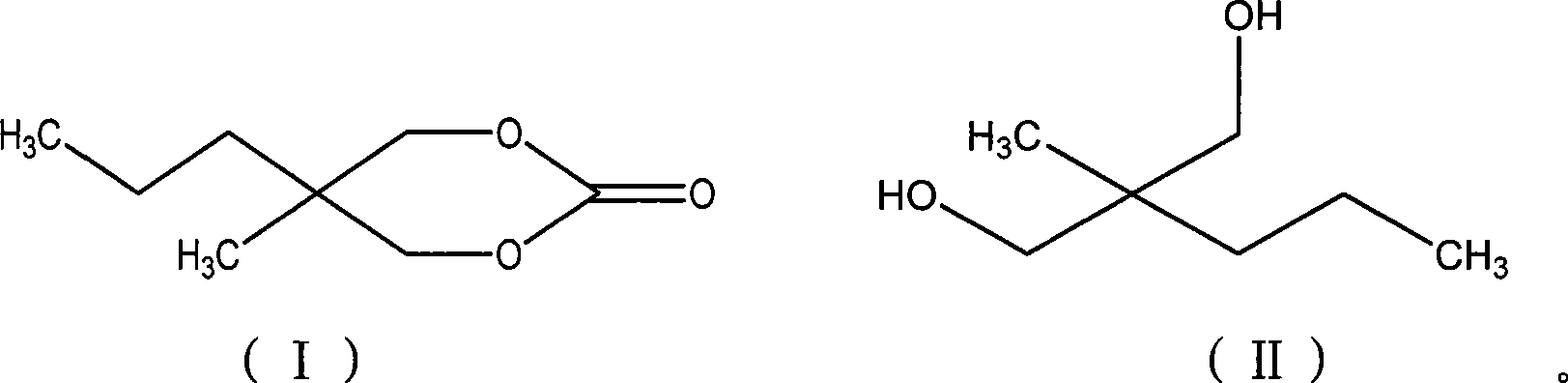
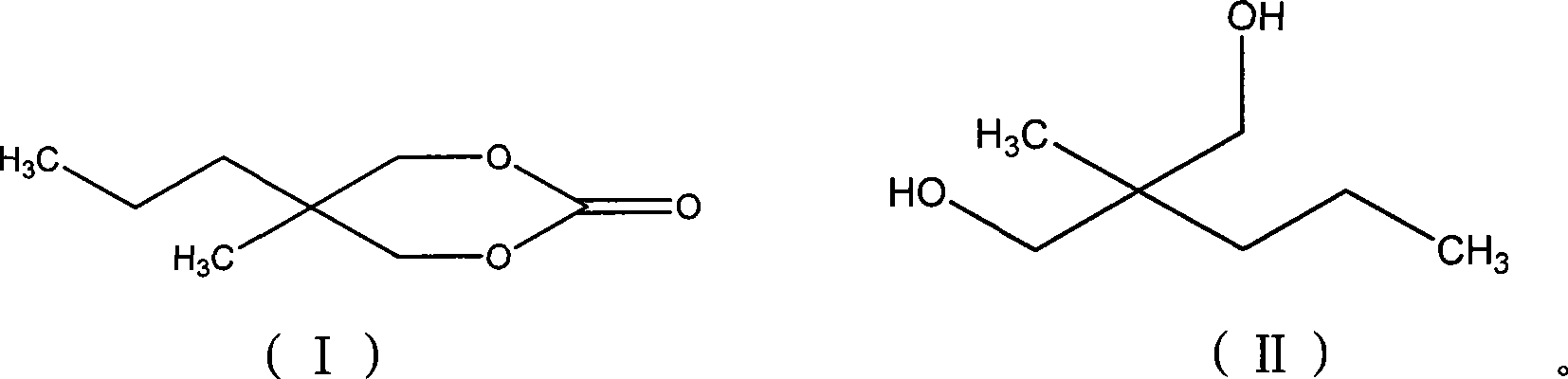
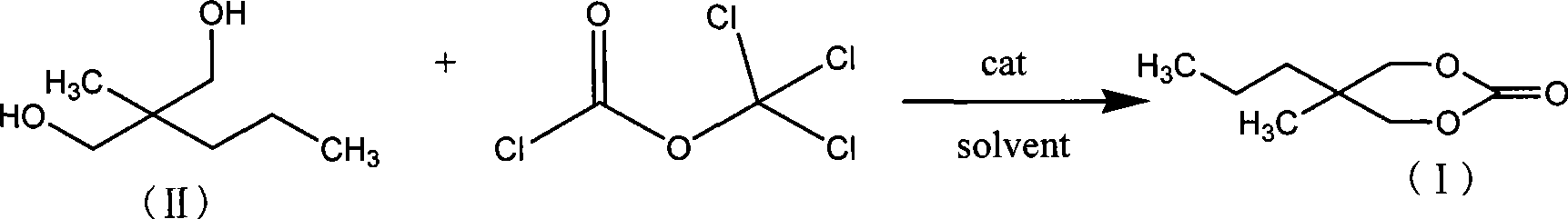
Method for synthesizing 2-methyl-2-propyl-1,3-propanediol dimethyl carbonate compound
A propylene glycol carbonate diester and synthesis method technology, applied in the direction of organic chemistry, can solve the problems of high sealing requirements of reaction equipment, troublesome phosgene environmental protection, and hidden safety hazards, and achieve the elimination of large safety hazards, low cost, and three wastes little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] According to 2-methyl-2-propyl-1,3-propanediol: trichloromethyl chloroformate: the molar ratio of organic amine catalyst substance is 1:0.5:0.2 feeding intake, 2-methyl-2-propyl-1 , 3-propanediol 30g; Trichloromethyl chloroformate 22.46g; Organic solvent chlorobenzene 120g; Organic amine catalyst is N, N-dimethylaniline, feeding quality 5.49g;
[0021] Dissolve 2-methyl-2-propyl-1,3-propanediol and organic amine catalyst in some organic solvents, cool down to -5~15°C, slowly add trichloromethyl chloroformate dissolved in a small amount of organic solvents dropwise Solution, react at 50 ℃ after completion of dropwise addition, track and monitor the reaction end point with gas chromatography, until the completion of the reaction, the chlorobenzene used to dissolve 2-methyl-2-propyl-1,3-propanediol and the organic amine catalyst and The total amount of organic solvent chlorobenzene for dissolving trichloromethyl chloroformate is 120g.
[0022] After the reaction is comple...
Embodiment 2
[0024] According to 2-methyl-2-propyl-1,3-propanediol: trichloromethyl chloroformate: the molar ratio of organic amine catalyst substance is 1:0.5:0.5 feeding intake, 2-methyl-2-propyl-1 , 3-propanediol 30g; Trichloromethyl chloroformate 22.46g; Organic solvent chlorobenzene 120g; Organic amine catalyst is N, N-dimethylaniline, feeding quality 13.73g;
[0025] The reaction was carried out at a temperature of 30° C., and other operations were the same as in Example 1 to obtain 25.20 g of 2-methyl-2-propyl-1,3-propanediol carbonate, with a yield of 70.2% and a purity of 98.1%.
Embodiment 3
[0027] According to 2-methyl-2-propyl-1,3-propanediol: trichloromethyl chloroformate: the molar ratio of organic amine catalyst substance is 1:0.7:0.5 feeding intake, 2-methyl-2-propyl-1 , 3-propanediol 30g; Trichloromethyl chloroformate 31.45g; Organic solvent cyclohexanone 180g; Organic amine catalyst is N, N-dimethylaniline, feeding quality 13.73g;
[0028] The reaction was carried out at -5°C, and other operations were the same as in Example 1 to obtain 28.07 g of 2-methyl-2-propyl-1,3-propanediol carbonate, with a yield of 82.1% and a purity of 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com