MUC1 tandem repeat sequences polypeptide, preparation technique thereof and use as anti-tumor medicament
A tandem repeat sequence and tandem repeat technology, applied in the field of tumor treatment, can solve the problems of limiting Th1 activation, tumor cell escape from the immune system, and direct effects that have not been reported in the literature to achieve the effect of inhibiting proliferation and growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
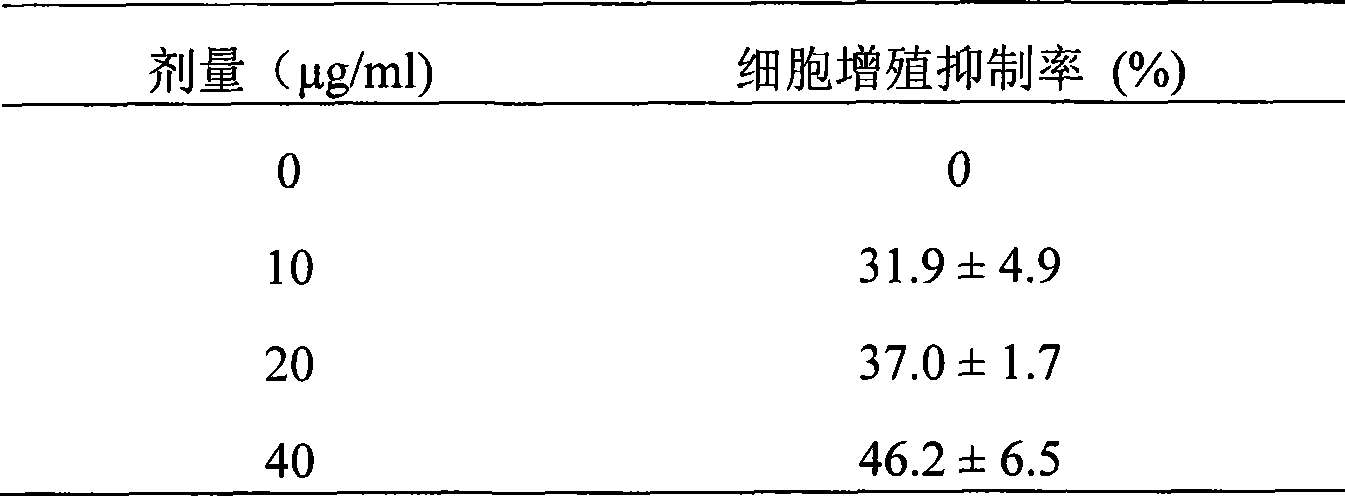
[0064] Synthesis of MUC1 tandem repeat polypeptide
[0065] A cDNA fragment of 1 to 10 tandem repeat sequences composed of 20 amino acid APDTRPAPGSTAPPAHGVTS of MUC1 tandem repeat sequence polypeptide was used to connect into pGEX-4T-1 vector, and then the recombinant plasmid was transformed into Escherichia coli DH5α. Transformed colonies were screened with ampicillin, and insert fragments were analyzed by enzyme digestion and gene sequencing. The recombinant plasmid was transformed into Escherichia coli DH5α, the expression was induced by 0.3mM IPTG, and the stable expression strain pGEX-MUC1 / DH5α was obtained by screening. A small amount of bacteria was boiled and lysed, the expression of MUC1-GST fusion protein was analyzed by 10% SDS-PAGE, and specific expression bands were identified by Western blotting. A large number of engineered bacteria were cultivated, and the supernatant was collected after sonication to purify human MUC1-GST fusion protein through Glutathione--S...
Embodiment 2
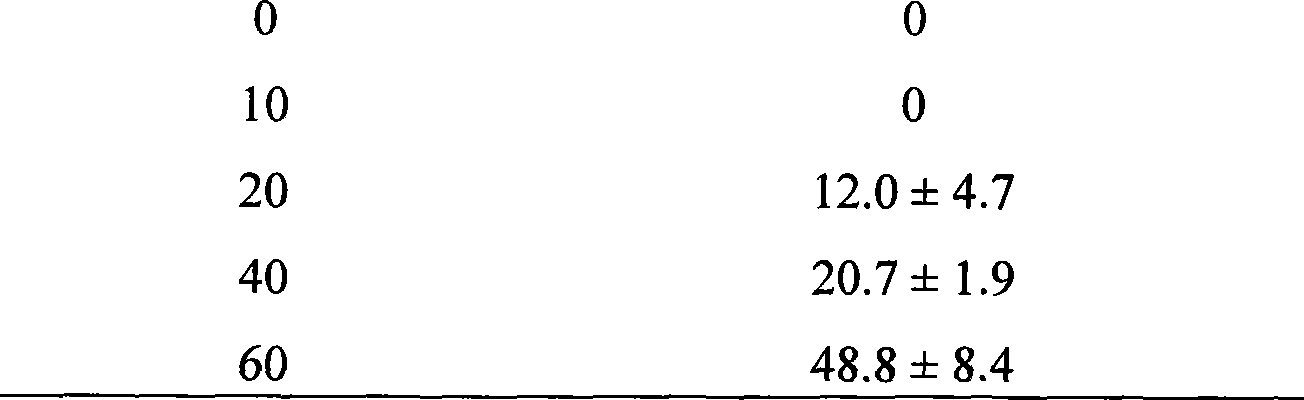
[0067] Synthesis of MUC1 tandem repeat polypeptide
[0068] Using the cDNA fragment of 1-10 tandem repeat sequences composed of 20 amino acid PPAHGVTSAPPDTRPAPGSTA of the MUC1 tandem repeat sequence polypeptide, the MUC1 polypeptide was synthesized according to the process described in Example 1, and made into a pharmaceutical preparation.
Embodiment 3
[0070] Synthesis of MUC1 tandem repeat polypeptide
[0071] Using the cDNA fragment of 1-10 tandem repeat sequences composed of 20 amino acids of the MUC1 tandem repeat sequence polypeptide DTRPAPGSTAPPAHGVTSAP, the MUC1 polypeptide was synthesized according to the process described in Example 1, and made into a pharmaceutical preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com